Renal excretion of recombinant immunotoxins containing pseudomonas exotoxin
- PMID: 21410247
- PMCID: PMC6290476
- DOI: 10.1021/bc1005152
Renal excretion of recombinant immunotoxins containing pseudomonas exotoxin
Abstract
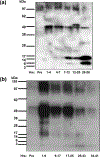
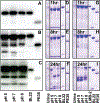
Recombinant immunotoxins BL22 (CAT-3888) and LMB-2, composed of Fv fragments of anti-CD22 and CD25 MAbs, respectively, have produced major responses in patients with hematologic malignancies, and are also associated with renal toxicity, particularly with BL22. Characterization of the renal excretion of recombinant immunotoxins, which have 2-4 h half-lives in plasma, has not been reported in humans. To study the renal excretion of recombinant immunotoxins, urine from patients treated with BL22 was collected and the recombinant protein visualized after trichloroacetic acid (TCA) precipitation or anion exchange chromatography. BL22 viewed by immunoblot was found in the urine of patients within 8 h after dosing as an intact protein, and progressively degraded to fragments of <20 kDa within 1 day. We studied the stability of BL22 and LMB-2 added to urine at different time points and pH. When exposed to urine ex vivo, BL22 time-dependent proteolysis was similar to that observed in treated patients. By N-terminal sequencing, proteolysis was documented at positions 348-349 and 350-351 of BL22, and 339-340 and 341-342 of LMB-2, and other proteolytic sites were observed as well. Our data suggest that BL22 is excreted into the urine in a potentially cytotoxic form, even after its plasma level declines, and may remain intact long enough to cause renal toxicity.
Figures



References
-
- Kreitman RJ, Wilson WH, Robbins D, Margulies I, Stetler-Stevenson M, Waldmann TA, and Pastan I (1999) Responses in refractory hairy cell leukemia to a recombinant immunotoxin. Blood 94, 3340–3348. - PubMed
-
- Kreitman RJ, Wilson WH, White JD, Stetler-Stevenson M, Jaffe ES, Waldmann TA, and Pastan I (2000) Phase I trial of recombinant immunotoxin Anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J. Clin. Oncol 18, 1614–1636. - PubMed
-
- Kreitman RJ, Wilson WH, Bergeron K, Raggio M, Stetler-Stevenson M, FitzGerald DJ, and Pastan I (2001) Efficacy of the Anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. New Engl. J. Med 345, 241–247. - PubMed
-
- Kreitman RJ, Squires DR, Stetler-Stevenson M, Noel P, Fitzgerald DJ, Wilson WH, and Pastan I (2005) Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J. Clin. Oncol 23, 6719–29. - PubMed
-
- Kreitman RJ, and Pastan I (2006) Immunotoxins in the treatment of refractory hairy cell leukemia. Hematol. Oncol. Clin. North Am 20, 1137–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

