Metabolomics reveals target and off-target toxicities of a model organophosphate pesticide to roach (Rutilus rutilus): implications for biomonitoring
- PMID: 21410251
- PMCID: PMC3076994
- DOI: 10.1021/es103814d
Metabolomics reveals target and off-target toxicities of a model organophosphate pesticide to roach (Rutilus rutilus): implications for biomonitoring
Abstract
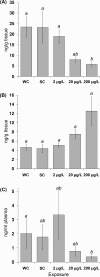
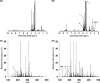
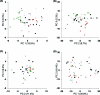
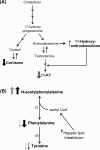
The ability of targeted and nontargeted metabolomics to discover chronic ecotoxicological effects is largely unexplored. Fenitrothion, an organophosphate pesticide, is categorized as a "red list" pollutant, being particularly hazardous to aquatic life. It acts primarily as a cholinesterase inhibitor, but evidence suggests it can also act as an androgen receptor antagonist. Whole-organism fenitrothion-induced toxicity is well-established, but information regarding target and off-target molecular toxicities is limited. Here we study the molecular responses of male roach ( Rutilus rutilus ) exposed to fenitrothion, including environmentally realistic concentrations, for 28 days. Acetylcholine was assessed in brain; steroid metabolism was measured in testes and plasma; and NMR and mass spectrometry-based metabolomics were conducted on testes and liver to discover off-target toxicity. O-demethylation was confirmed as a major route of pesticide degradation. Fenitrothion significantly depleted acetylcholine, confirming its primary mode of action, and 11-ketotestosterone in plasma and cortisone in testes, showing disruption of steroid metabolism. Metabolomics revealed significant perturbations to the hepatic phosphagen system and previously undocumented effects on phenylalanine metabolism in liver and testes. On the basis of several unexpected molecular responses that were opposite to the anticipated acute toxicity, we propose that chronic pesticide exposure induces an adapting phenotype in roach, which may have considerable implications for interpreting molecular biomarker responses in field-sampled fish.
Figures

References
-
- U.K. Environment Agency. Pollution Inventory Substances: Fenitrothion. http://www.environment-agency.gov.uk/business/topics/pollution/158.aspx, 2010.
-
- Fait G.; Nicelli M.; Fragoulis G.; Trevisan M.; Capri E. Reduction of point contamination sources of pesticide from a vineyard farm. Environ. Sci. Technol. 2007, 41, 3302–3308. - PubMed
-
- Tanabe A.; Kawata K. Daily variation of pesticides in surface water of a small river flowing through paddy field area. Bull. Environ. Contam. Toxicol. 2009, 82, 705–710. - PubMed
-
- U.S. Environmental Protection Agency. Fenitrothion Final Work Plan Registration Review: July 2009. http://www.regulations.gov/search/Regs/home.html#documentDetail?R=090000..., 2009.
-
- Crathorne B.; Dobbs A. J., Chemical pollution of the aquatic environment by priority pollutants and its control. In Pollution: Causes, Effects and Control, 2nd ed.; Harrison R. M., Ed.; Royal Society of Chemistry: Cambridge, U.K., 1990; pp 1−18.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

