Pharmacokinetic profile of lesogaberan (AZD3355) in healthy subjects: a novel GABA(B)-receptor agonist reflux inhibitor
- PMID: 21410297
- PMCID: PMC3585951
- DOI: 10.2165/11590310-000000000-00000
Pharmacokinetic profile of lesogaberan (AZD3355) in healthy subjects: a novel GABA(B)-receptor agonist reflux inhibitor
Abstract
Background: Lesogaberan (AZD3355) is a novel reflux inhibitor developed as an add-on treatment to proton pump inhibitors (PPIs) for symptom relief in patients with gastroesophageal reflux disease who have a partial response to PPI therapy.
Objective: The aim of this study was to evaluate the pharmacokinetic profile of lesogaberan in healthy subjects after single oral and intravenous administration of (14)C-labeled lesogaberan and non-(14)C-labeled lesogaberan.
Study design: This was an open-label, single-center, randomized, two-way crossover, phase I study.
Participants: Ten healthy male subjects took part in the study.
Intervention: Volunteers were randomized to receive a single dose of either orally dosed (100 mg) or intravenously infused (20 mg) non-(14)C-labeled lesogaberan, and then orally (100 mg) or intravenously (20 mg) administered (14)C-labeled lesogaberan in a crossover design. Treatment periods were separated by a washout period of at least 7 days.
Main outcome measures: Analyses of the rate and route of excretion, dose recovery, area under the plasma concentration versus time curve (AUC), AUC to the last quantifiable concentration, maximal plasma concentration (C(max)), time to C(max), the apparent elimination half-life, bioavailability, total clearance, renal clearance, fraction of the bioavailable dose excreted unchanged in the urine, cumulative amount of drug excreted unchanged in urine, and the apparent volume of distribution at steady state of lesogaberan.
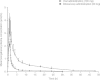
Results: Lesogaberan was rapidly and extensively absorbed from the gastrointestinal tract and C(max) was achieved within 1-2 hours of oral dosing. The terminal half-life of lesogaberan was between 11 and 13 hours. Renal clearance accounted for approximately 22% of total body clearance. Based on the recovery of administered radioactivity, approximately 84% of the dose was excreted into the urine either as the parent compound or as water-soluble metabolite(s). There were no safety concerns raised during the study.
Conclusion: Orally administered lesogaberan is rapidly absorbed with high bioavailability and the majority of the dose is excreted by the kidneys either as the parent compound or as metabolites. The major elimination pathway for lesogaberan in man is metabolism.
Figures





References
-
- Lehmann A, Antonsson M, Holmberg AA, et al. (R)-(3-amino-2-fluoropropyl) phosphinic acid (AZD3355), a novel GABAB receptor agonist, inhibits transient lower esophageal sphincter relaxation through a peripheral mode of action. J Pharmacol Exp Ther. 2009;331(2):504–12. doi: 10.1124/jpet.109.153593. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
