Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children
- PMID: 21410369
- PMCID: PMC3093964
- DOI: 10.1056/NEJMoa1009705
Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children
Abstract
Background: Research has underscored the effects of exposure and sensitization to allergens on the severity of asthma in inner-city children. It has also revealed the limitations of environmental remediation and guidelines-based therapy in achieving greater disease control.
Methods: We enrolled inner-city children, adolescents, and young adults with persistent asthma in a randomized, double-blind, placebo-controlled, parallel-group trial at multiple centers to assess the effectiveness of omalizumab, as compared with placebo, when added to guidelines-based therapy. The trial was conducted for 60 weeks, and the primary outcome was symptoms of asthma.
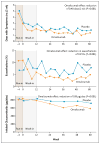
Results: Among 419 participants who underwent randomization (at which point 73% had moderate or severe disease), omalizumab as compared with placebo significantly reduced the number of days with asthma symptoms, from 1.96 to 1.48 days per 2-week interval, a 24.5% decrease (P<0.001). Similarly, omalizumab significantly reduced the proportion of participants who had one or more exacerbations from 48.8 to 30.3% (P<0.001). Improvements occurred with omalizumab despite reductions in the use of inhaled glucocorticoids and long-acting beta-agonists.
Conclusions: When added to a regimen of guidelines-based therapy for inner-city children, adolescents, and young adults, omalizumab further improved asthma control, nearly eliminated seasonal peaks in exacerbations, and reduced the need for other medications to control asthma. (Funded by the National Institute of Allergy and Infectious Diseases and Novartis; ClinicalTrials.gov number, NCT00377572.).
Figures

Comment in
-
Anti-IgE for asthma in inner-city children.N Engl J Med. 2011 Jun 30;364(26):2557; author reply 2557-8. doi: 10.1056/NEJMc1104264. N Engl J Med. 2011. PMID: 21714663 No abstract available.
-
Anti-IgE for asthma in inner-city children.N Engl J Med. 2011 Jun 30;364(26):2556-7; author reply 2557-8. doi: 10.1056/NEJMc1104264. N Engl J Med. 2011. PMID: 21714664 No abstract available.
-
Omalizumab reduces frequency of asthma exacerbations in children.J Pediatr. 2011 Sep;159(3):512-3. doi: 10.1016/j.jpeds.2011.07.004. J Pediatr. 2011. PMID: 21846525 No abstract available.
References
-
- Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–80. - PubMed
-
- Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. - PubMed
-
- Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–90. - PubMed
-
- Fahy JV, Fleming HE, Wong HH, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155:1828–34. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- N01-AI-25482/AI/NIAID NIH HHS/United States
- N01 AI025496/AI/NIAID NIH HHS/United States
- M01RR00533/RR/NCRR NIH HHS/United States
- U54 HD061221/HD/NICHD NIH HHS/United States
- M01 RR000071/RR/NCRR NIH HHS/United States
- 1UL1RR025771/RR/NCRR NIH HHS/United States
- 5M01RR020359-040/RR/NCRR NIH HHS/United States
- M01RR00071/RR/NCRR NIH HHS/United States
- 1UL1RR024156/RR/NCRR NIH HHS/United States
- N01 AI025482/AI/NIAID NIH HHS/United States
- M01 RR000533/RR/NCRR NIH HHS/United States
- UL1 RR024156/RR/NCRR NIH HHS/United States
- P30 DK027651/DK/NIDDK NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- N01-AI-25496/AI/NIAID NIH HHS/United States
- M01 RR020359/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
