Efficacy and safety of sirolimus in lymphangioleiomyomatosis
- PMID: 21410393
- PMCID: PMC3118601
- DOI: 10.1056/NEJMoa1100391
Efficacy and safety of sirolimus in lymphangioleiomyomatosis
Abstract
Background: Lymphangioleiomyomatosis (LAM) is a progressive, cystic lung disease in women; it is associated with inappropriate activation of mammalian target of rapamycin (mTOR) signaling, which regulates cellular growth and lymphangiogenesis. Sirolimus (also called rapamycin) inhibits mTOR and has shown promise in phase 1-2 trials involving patients with LAM.
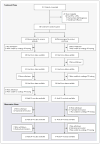
Methods: We conducted a two-stage trial of sirolimus involving 89 patients with LAM who had moderate lung impairment--a 12-month randomized, double-blind comparison of sirolimus with placebo, followed by a 12-month observation period. The primary end point was the difference between the groups in the rate of change (slope) in forced expiratory volume in 1 second (FEV(1)).
Results: During the treatment period, the FEV(1) slope was -12±2 ml per month in the placebo group (43 patients) and 1±2 ml per month in the sirolimus group (46 patients) (P<0.001). The absolute between-group difference in the mean change in FEV(1) during the treatment period was 153 ml, or approximately 11% of the mean FEV(1) at enrollment. As compared with the placebo group, the sirolimus group had improvement from baseline to 12 months in measures of forced vital capacity, functional residual capacity, serum vascular endothelial growth factor D (VEGF-D), and quality of life and functional performance. There was no significant between-group difference in this interval in the change in 6-minute walk distance or diffusing capacity of the lung for carbon monoxide. After discontinuation of sirolimus, the decline in lung function resumed in the sirolimus group and paralleled that in the placebo group. Adverse events were more common with sirolimus, but the frequency of serious adverse events did not differ significantly between the groups.
Conclusions: In patients with LAM, sirolimus stabilized lung function, reduced serum VEGF-D levels, and was associated with a reduction in symptoms and improvement in quality of life. Therapy with sirolimus may be useful in selected patients with LAM. (Funded by the National Institutes of Health and others; MILES ClinicalTrials.gov number, NCT00414648.).
Figures

Comment in
-
Patient organizations and research on rare diseases.N Engl J Med. 2011 Apr 28;364(17):1670-1. doi: 10.1056/NEJMe1102290. Epub 2011 Mar 16. N Engl J Med. 2011. PMID: 21410388 No abstract available.
-
Efficacy and safety of sirolimus in lymphangioleiomyomatosis.N Engl J Med. 2011 Jul 21;365(3):271-2; author reply 272. doi: 10.1056/NEJMc1106358. N Engl J Med. 2011. PMID: 21774717 No abstract available.
-
Efficacy and safety of sirolimus in lymphangioleiomyomatosis.N Engl J Med. 2011 Jul 21;365(3):271; author reply 272. doi: 10.1056/NEJMc1106358. N Engl J Med. 2011. PMID: 21774718 No abstract available.
References
-
- Taveira-DaSilva AM, Steagall WK, Moss J. Lymphangioleiomyomatosis. Cancer Control. 2006;13:276–85. - PubMed
-
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest. 2008;133:507–16. - PubMed
-
- Urban T, Lazor R, Lacronique J, et al. Pulmonary lymphangioleiomyomatosis: a study of 69 patients. Medicine (Baltimore) 1999;78:321–37. - PubMed
-
- Johnson SR, Tattersfield AE. Decline in lung function in lymphangioleiomyomatosis: relation to menopause and progesterone treatment. Am J Respir Crit Care Med. 1999;160:628–33. - PubMed
-
- Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Hathaway O, Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126:1867–74. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
