Bone resorption and remodeling in murine collagenase-induced osteoarthritis after administration of glucosamine
- PMID: 21410959
- PMCID: PMC3132029
- DOI: 10.1186/ar3283
Bone resorption and remodeling in murine collagenase-induced osteoarthritis after administration of glucosamine
Abstract
Introduction: Glucosamine is an amino-monosaccharide and precursor of glycosaminoglycans, major components of joint cartilage. Glucosamine has been clinically introduced for the treatment of osteoarthritis but the data about its protective role in disease are insufficient. The goal of this study was to investigate the effect of long term administration of glucosamine on bone resorption and remodeling.
Methods: The effect of glucosamine on bone resorption and remodeling was studied in a model of collagenase-induced osteoarthritis (CIOA). The levels of macrophage-inflammatory protein (MIP)-1α, protein regulated upon activation, normal T-cell expressed, and secreted (RANTES), soluble receptor activator of nuclear factor kappa-B ligand (RANKL), tumor necrosis factor (TNF)-α, and interleukin (IL)-6, 4 and 10 in synovial fluid were measured by enzyme-linked immunosorbent assay (ELISA). Cell populations in synovial extracts and the expression of RANKL, of receptors for TNF-α (TNF-αR) and interferon γ (IFN-γR) on clusters of differentiation (CD) three positive T cells were analyzed by flow cytometry. Transforming growth factor (TGF)-β3, bone morphogenetic protein (BMP)-2, phosphorylated protein mothers against decapentaplegic homolog 2 (pSMAD-2), RANKL and Dickkopf-1 protein (DKK-1) positive staining in CIOA joints were determined by immunohistochemistry.
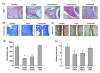
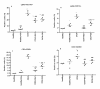
Results: The administration of glucosamine hydrochloride in CIOA mice inhibited loss of glycosaminoglycans (GAGs) and proteoglycans (PGs) in cartilage, bone erosion and osteophyte formation. It decreased the levels of soluble RANKL and IL-6 and induced IL-10 increase in the CIOA joint fluids. Glucosamine limited the number of CD11b positive Ly6G neutrophils and RANKL positive CD3 T cells in the joint extracts. It suppressed bone resorption via down-regulation of RANKL expression and affected bone remodeling in CIOA by decreasing BMP-2, TGF-β3 and pSMAD-2 expression and up-regulating DKK-1 joint levels.
Conclusions: Our data suggest that glucosamine hydrochloride inhibits bone resorption through down-regulation of RANKL expression in the joints, via reduction of the number of RANKL positive CD3 T cells and the level of sRANKL in the joints extracts. These effects of glucosamine appear to be critical for the progression of CIOA and result in limited bone remodeling of the joints.
Figures
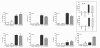
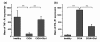


Similar articles
-
Involvement of soluble receptor activator of nuclear factor-κB ligand (sRANKL) in collagenase-induced murine osteoarthritis and human osteoarthritis.Rheumatol Int. 2012 May;32(5):1317-25. doi: 10.1007/s00296-010-1723-8. Epub 2011 Feb 3. Rheumatol Int. 2012. PMID: 21290130
-
The role of NOX2-derived reactive oxygen species in collagenase-induced osteoarthritis.Osteoarthritis Cartilage. 2018 Dec;26(12):1722-1732. doi: 10.1016/j.joca.2018.08.014. Epub 2018 Sep 5. Osteoarthritis Cartilage. 2018. PMID: 30195046
-
Interleukin-1 is not involved in synovial inflammation and cartilage destruction in collagenase-induced osteoarthritis.Osteoarthritis Cartilage. 2017 Mar;25(3):385-396. doi: 10.1016/j.joca.2016.09.009. Epub 2016 Sep 18. Osteoarthritis Cartilage. 2017. PMID: 27654963
-
RANK, RANKL and osteoprotegerin in arthritic bone loss.Braz J Med Biol Res. 2005 Feb;38(2):161-70. doi: 10.1590/s0100-879x2005000200004. Epub 2005 Feb 15. Braz J Med Biol Res. 2005. PMID: 15785827 Review.
-
Bone Remodeling in Osteoarthritis-Biological and Radiological Aspects.Medicina (Kaunas). 2023 Sep 7;59(9):1613. doi: 10.3390/medicina59091613. Medicina (Kaunas). 2023. PMID: 37763732 Free PMC article. Review.
Cited by
-
Is there any scientific evidence for the use of glucosamine in the management of human osteoarthritis?Arthritis Res Ther. 2012 Jan 30;14(1):201. doi: 10.1186/ar3657. Arthritis Res Ther. 2012. PMID: 22293240 Free PMC article. Review.
-
Wnt5a/Ror2 mediates temporomandibular joint subchondral bone remodeling.J Dent Res. 2015 Jun;94(6):803-12. doi: 10.1177/0022034515576051. Epub 2015 Mar 6. J Dent Res. 2015. PMID: 25749876 Free PMC article.
-
Pleiotropic Effects of Metformin in Osteoarthritis.Life (Basel). 2023 Feb 3;13(2):437. doi: 10.3390/life13020437. Life (Basel). 2023. PMID: 36836794 Free PMC article. Review.
-
Chondroitin and glucosamine in the management of osteoarthritis: an update.Curr Rheumatol Rep. 2013 Oct;15(10):361. doi: 10.1007/s11926-013-0361-z. Curr Rheumatol Rep. 2013. PMID: 23955063 Review.
-
Emerging pharmaceutical therapeutics and delivery technologies for osteoarthritis therapy.Front Pharmacol. 2022 Nov 17;13:945876. doi: 10.3389/fphar.2022.945876. eCollection 2022. Front Pharmacol. 2022. PMID: 36467045 Free PMC article. Review.
References
-
- Traxinger RR, Marshall S. Coordinated regulation of glutamine:fructose-6-phosphate amidotransferase activity by insulin, glucose, and glutamine. Role of hexosamine biosynthesis in enzyme regulation. J Biol Chem. 1991;266:10148–10154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

