Cytochrome P450: taming a wild type enzyme
- PMID: 21411308
- PMCID: PMC3118264
- DOI: 10.1016/j.copbio.2011.02.008
Cytochrome P450: taming a wild type enzyme
Abstract
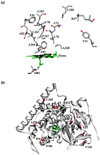
Protein engineering of cytochrome P450 monooxygenases (P450s) has been very successful in generating valuable non-natural activities and properties, allowing these powerful catalysts to be used for the synthesis of drug metabolites and in biosynthetic pathways for the production of precursors of artemisinin and paclitaxel. Collected experience indicates that the P450s are highly 'evolvable' - they are particularly robust to mutation in their active sites and readily accept new substrates and exhibit new selectivities. Their ability to adapt to new challenges upon mutation may reflect the nonpolar nature of their active sites as well as their high degree of conformational variability.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors are aware of no conflicts of interest regarding the preparation and submission of this manuscript.
Figures
References
-
- Dewick PM. Medicinal Natural Products: A Biosynthetic Approach. 3rd Edition. John Wiley & Sons; 2009.
-
- Gillam EMJ. Engineering cytochrome P450 Enzymes. Chem Res Toxicol. 2008;21:220–231. - PubMed
-
- Grogan G. Cytochromes P450: exploiting diversity and enabling application as biocatalysts. Curr Opin Chem Biol. 2011 article in press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

