A new Ebola virus nonstructural glycoprotein expressed through RNA editing
- PMID: 21411529
- PMCID: PMC3094950
- DOI: 10.1128/JVI.02190-10
A new Ebola virus nonstructural glycoprotein expressed through RNA editing
Abstract
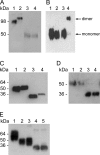
Ebola virus (EBOV), an enveloped, single-stranded, negative-sense RNA virus, causes severe hemorrhagic fever in humans and nonhuman primates. The EBOV glycoprotein (GP) gene encodes the nonstructural soluble glycoprotein (sGP) but also produces the transmembrane glycoprotein (GP₁,₂) through transcriptional editing. A third GP gene product, a small soluble glycoprotein (ssGP), has long been postulated to be produced also as a result of transcriptional editing. To identify and characterize the expression of this new EBOV protein, we first analyzed the relative ratio of GP gene-derived transcripts produced during infection in vitro (in Vero E6 cells or Huh7 cells) and in vivo (in mice). The average percentages of transcripts encoding sGP, GP₁,₂, and ssGP were approximately 70, 25, and 5%, respectively, indicating that ssGP transcripts are indeed produced via transcriptional editing. N-terminal sequence similarity with sGP, the absence of distinguishing antibodies, and the abundance of sGP made it difficult to identify ssGP through conventional methodology. Optimized 2-dimensional (2D) gel electrophoresis analyses finally verified the expression and secretion of ssGP in tissue culture during EBOV infection. Biochemical analysis of recombinant ssGP characterized this protein as a disulfide-linked homodimer that was exclusively N glycosylated. In conclusion, we have identified and characterized a new EBOV nonstructural glycoprotein, which is expressed as a result of transcriptional editing of the GP gene. While ssGP appears to share similar structural properties with sGP, it does not appear to have the same anti-inflammatory function on endothelial cells as sGP.
Figures


References
-
- Barrientos L. G., Martin A. M., Rollin P. E., Sanchez A. 2004. Disulfide bond assignment of the Ebola virus secreted glycoprotein SGP. Biochem. Biophys. Res. Commun. 323:696–702 - PubMed
-
- Barrientos L. G., Martin A. M., Wohlhueter R. M., Rollin P. E. 2007. Secreted glycoprotein from live Zaire ebolavirus-infected cultures: preparation, structural and biophysical characterization, and thermodynamic stability. J. Infect. Dis. 196(Suppl. 2):S220–S231 - PubMed
-
- Bourhis J. M., Canard B., Longhi S. 2006. Structural disorder within the replicative complex of measles virus: functional implications. Virology 344:94–110 - PubMed
-
- Bray M., Davis K., Geisbert T., Schmaljohn C., Huggins J. 1998. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J. Infect. Dis. 178:651–661 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

