Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing
- PMID: 21411758
- PMCID: PMC3357943
- DOI: 10.1182/blood-2011-01-330720
Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing
Abstract
Bone marrow-derived cells (BMDCs) contribute to postnatal vascular growth by differentiating into endothelial cells or secreting angiogenic factors. However, the extent of their endothelial differentiation highly varies according to the angiogenic models used. Wound healing is an intricate process in which the skin repairs itself after injury. As a process also observed in cancer progression, neoangiogenesis into wound tissues is profoundly involved in this healing process, suggesting the contribution of BMDCs. However, the extent of the differentiation of BMDCs to endothelial cells in wound healing is unclear. In this study, using the green fluorescent protein-bone marrow chim-eric experiment and high resolution confocal microscopy at a single cell level, we observed no endothelial differentiation of BMDCs in 2 acute wound healing models (dorsal excisional wound and ear punch) and a chronic wound healing model (decubitus ulcer). Instead, a major proportion of BMDCs were macrophages. Indeed, colony-stimulating factor 1 (CSF-1) inhibition depleted approximately 80% of the BMDCs at the wound healing site. CSF-1-mutant (CSF-1(op/op)) mice showed significantly reduced neoangiogenesis into the wound site, supporting the substantial role of BMDCs as macrophages. Our data show that the proangiogenic effects of macrophages, but not the endothelial differentiation, are the major contribution of BMDCs in wound healing.
Figures
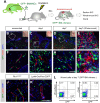
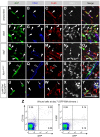

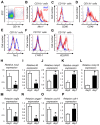
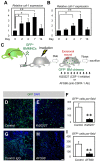
References
-
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. - PubMed
-
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. - PubMed
-
- Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6(11):835–845. - PubMed
-
- Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319(5860):195–198. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

