Inconsistent immunohistochemical expression patterns of four different CD133 antibody clones in glioblastoma
- PMID: 21411810
- PMCID: PMC3201141
- DOI: 10.1369/0022155411400867
Inconsistent immunohistochemical expression patterns of four different CD133 antibody clones in glioblastoma
Abstract
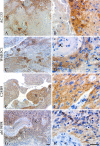
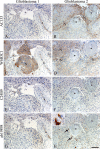
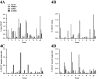
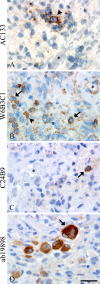
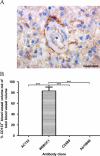
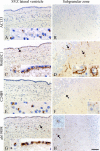
The putative tumor stem cell marker CD133 is the marker of choice for identifying brain tumor stem cells in gliomas, but the use of different CD133 antibody clones possibly recognizing different CD133 splice variants with epitopes of different glycosylation status confuses the field. The aim was to investigate if current inconsistent CD133 observations could be a result of using different CD133 antibodies for immunohistochemical identification of CD133. Ten glioblastomas were immunohistochemically stained with four different CD133 antibody clones (AC133, W6B3C1, C24B9, and ab19898) and analyzed by quantitative stereology. Moreover, the CD133 staining pattern of each antibody clone was investigated in kidney, pancreas, and placenta tissue as well as in glioblastoma and retinoblastoma cultures and cell lines. All antibody clones revealed CD133+ niches and single cells in glioblastomas, but when using different clones, their distribution rarely corresponded. Morphology of identified single cells varied, and staining of various tissues, cultures, and cells lines was also inconsistent among the clones. In conclusion, the authors report inconsistent CD133 detection when using different primary CD133 antibody clones. Thus, direct comparison of studies using different antibody clones and conclusions based on CD133 immunohistochemistry should be performed with caution.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures



References
-
- Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. 2007. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 67:4010–4015 - PubMed
-
- Brehar FM, Bleotu C, Stefan LM, Buzgariu W, Chivu M, Utoiu E, Matei L, Ciurea AV, Tascu A. 2009. Isolation and partial characterization of a new human glioblastoma cell line. Chirurgia. 104:453–461 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

