Sensory innervation of the nonspecialized connective tissues in the low back of the rat
- PMID: 21411968
- PMCID: PMC3238034
- DOI: 10.1159/000323875
Sensory innervation of the nonspecialized connective tissues in the low back of the rat
Abstract
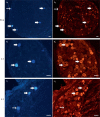
Chronic musculoskeletal pain, including low back pain, is a worldwide debilitating condition; however, the mechanisms that underlie its development remain poorly understood. Pathological neuroplastic changes in the sensory innervation of connective tissue may contribute to the development of nonspecific chronic low back pain. Progress in understanding such potentially important abnormalities is hampered by limited knowledge of connective tissue's normal sensory innervation. The goal of this study was to evaluate and quantify the sensory nerve fibers terminating within the nonspecialized connective tissues in the low back of the rat. With 3-dimensional reconstructions of thick (30-80 μm) tissue sections we have for the first time conclusively identified sensory nerve fiber terminations within the collagen matrix of connective tissue in the low back. Using dye labeling techniques with Fast Blue, presumptive dorsal root ganglia cells that innervate the low back were identified. Of the Fast Blue-labeled cells, 60-88% also expressed calcitonin gene-related peptide (CGRP) immunoreactivity. Based on the immunolabeling with CGRP and the approximate size of these nerve fibers (≤2 μm) we hypothesize that they are Aδ or C fibers and thus may play a role in the development of chronic pain.
Copyright © 2011 S. Karger AG, Basel.
Figures





References
-
- Bednar D.A., Orr F.W., S. G.T. Observations on the pathomorphology of the thoracolumbar fascia in chronic mechanical back pain: a microscopic study. Spine. 1995;20:1161–1164. - PubMed
-
- Bjur D., Alfredson H., Forsgren S. The innervation pattern of the human Achilles tendon: studies of the normal and tendinosis tendon with markers for general and sensory innervation. Cell Tissue Res. 2005;320:201–206. - PubMed
-
- Borg-Stein J., Wilkins A. Soft tissue determinants of low back pain. Curr Pain Headache Rep. 2006;10:339–344. - PubMed
-
- Bove G.M., Light A.R. Unmyelinated nociceptors of rat paraspinal tissues. J Neurophysiol. 1995;73:1752–1762. - PubMed
-
- Box G., Hunter W., Hunter J. Statistics for Experimenters. New York: Wiley; 1978.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

