CD4 cell eligibility thresholds: an analysis of the time to antiretroviral treatment in HIV-1 seroconverters
- PMID: 21412060
- PMCID: PMC3921664
- DOI: 10.1097/QAD.0b013e32834625d3
CD4 cell eligibility thresholds: an analysis of the time to antiretroviral treatment in HIV-1 seroconverters
Abstract
Background: WHO recommends initiating combination antiretroviral treatment at the minimal CD4 cell threshold of 350 cells/μl. In sub-Saharan Africa, the time for a recently infected patient to reach this threshold is unclear.
Method: We estimated the probability of reaching different CD4 cell thresholds over time in the ANRS 1220 cohort of HIV-1 seroconverters in Côte d'Ivoire. CD4 cell slopes were estimated using a mixed linear model. Probabilities of crossing the 350 and 500 cells/μl CD4 cell thresholds were estimated by the Kaplan-Meier method.
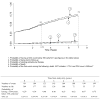
Results: Between 1997 and 2009, 304 recent seroconverters have been enrolled in the Primo-CI cohort (62% men, median baseline age 29 years and median time since the estimated date of seroconversion 9 months). The probability of having a first CD4 cell count below 500 cells/μl was 0.57, 0.72, 0.79 and 0.84 at study entry, 2, 4 and 6 years, respectively. For a first CD4 cell count below 350 cells/μl, these figures were 0.29, 0.40, 0.55 and 0.67. The time for 75% of patients to reach the threshold was 3.0 years for 500 cells/μl and 7.0 years for 350 cells/μl.
Figures


References
-
- WHO. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. Progress report. 2010 Available at: http://www.who.int/hiv/pub/2010progressreport/en/
-
- Granich R, Gilks C, Dye C, De Cock K, Williams B. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. - PubMed
-
- The ART-LINC Collaboration of International Databases to Evaluate AIDS (IeDEA) Keiser O, Anastos K, Schechter M, Balestre E, Boulle A, et al. Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13:870–9. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

