Dysferlin, annexin A1, and mitsugumin 53 are upregulated in muscular dystrophy and localize to longitudinal tubules of the T-system with stretch
- PMID: 21412170
- PMCID: PMC6309232
- DOI: 10.1097/NEN.0b013e31821350b0
Dysferlin, annexin A1, and mitsugumin 53 are upregulated in muscular dystrophy and localize to longitudinal tubules of the T-system with stretch
Abstract
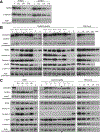
Mutations in dysferlin cause an inherited muscular dystrophy because of defective membrane repair. Three interacting partners of dysferlin are also implicated in membrane resealing: caveolin-3 (in limb girdle muscular dystrophy type 1C), annexin A1, and the newly identified protein mitsugumin 53 (MG53). Mitsugumin 53 accumulates at sites of membrane damage, and MG53-knockout mice display a progressive muscular dystrophy. This study explored the expression and localization of MG53 in human skeletal muscle, how membrane repair proteins are modulated in various forms of muscular dystrophy, and whether MG53 is a primary cause of human muscle disease. Mitsugumin 53 showed variable sarcolemmal and/or cytoplasmic immunolabeling in control human muscle and elevated levels in dystrophic patients. No pathogenic MG53 mutations were identified in 50 muscular dystrophy patients, suggesting that MG53 is unlikely to be a common cause of muscular dystrophy in Australia. Western blot analysis confirmed upregulation of MG53, as well as of dysferlin, annexin A1, and caveolin-3 to different degrees, in different muscular dystrophies. Importantly, MG53, annexin A1, and dysferlin localize to the t-tubule network and show enriched labeling at longitudinal tubules of the t-system in overstretch. Our results suggest that longitudinal tubules of the t-system may represent sites of physiological membrane damage targeted by this membrane repair complex.
Figures




References
-
- Bansal D, Miyake K, Vogel SS, et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 2003:423;168–72 - PubMed
-
- Matsuda C, Hayashi YK, Ogawa M, et al. The sarcolemmal proteins dysferlin and caveolin-3 interact in skeletal muscle. Hum Mol Genet 2001:10;1761–6 - PubMed
-
- Woodman SE, Sotgia F, Galbiati F, et al. Caveolinopathies: mutations in caveolin-3 cause four distinct autosomal dominant muscle diseases. Neurology 2004:62;538–43 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

