Lentiviral vectors for induction of self-differentiation and conditional ablation of dendritic cells
- PMID: 21412283
- PMCID: PMC3155152
- DOI: 10.1038/gt.2011.15
Lentiviral vectors for induction of self-differentiation and conditional ablation of dendritic cells
Abstract
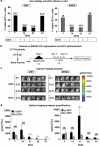
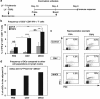
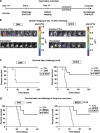
Development of lentiviral vectors (LVs) in the field of immunotherapy and immune regeneration will strongly rely on biosafety of the gene transfer. We demonstrated previously the feasibility of ex vivo genetic programming of mouse bone marrow precursors with LVs encoding granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4), which induced autonomous differentiation of long-lived dendritic cells (DCs), referred to as self-differentiated myeloid-derived antigen-presenting-cells reactive against tumors (SMART-DCs). Here, LV biosafety was enhanced by using a DC-restricted and physiological promoter, the major histocompatibility complex (MHC) II promoter, and including co-expression of the herpes simplex virus-thymidine kinase (sr39HSV-TK) conditional suicide gene. Tricistronic vectors co-expressing sr39HSV-TK, GM-CSF and IL-4 transcriptionally regulated by the MHCII promoter or the ubiquitous cytomegalovirus (CMV) promoter were compared. Despite the different gene transfer effects, such as the kinetics, levels of transgene expression and persistency of integrated vector copies, both vectors induced highly viable SMART-DCs, which persisted for at least 70 days in vivo and could be ablated with the pro-drug Ganciclovir (GCV). SMART-DCs co-expressing the tyrosine-related protein 2 melanoma antigen administered subcutaneously generated antigen-specific, anti-melanoma protective and therapeutic responses in the mouse B16 melanoma model. GCV administration after immunotherapy did not abrogate DC vaccination efficacy. This demonstrates proof-of-principle of genetically programmed DCs that can be ablated pharmacologically.
Figures






References
-
- Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. - PubMed
-
- Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. - PubMed
-
- Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. - PMC - PubMed
-
- Palucka AK, Dhodapkar MV, Paczesny S, Ueno H, Fay J, Banchereau J. Boosting vaccinations with peptide-pulsed CD34+ progenitor-derived dendritic cells can expand long-lived melanoma peptide-specific CD8+ T cells in patients with metastatic melanoma. J Immunother. 2005;28:158–168. - PubMed
-
- Banchereau J, Ueno H, Dhodapkar M, Connolly J, Finholt JP, Klechevsky E, et al. Immune and clinical outcomes in patients with stage IV melanoma vaccinated with peptide-pulsed dendritic cells derived from CD34+ progenitors and activated with type I interferon. J Immunother. 2005;28:505–516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

