AAV-mediated photoreceptor transduction of the pig cone-enriched retina
- PMID: 21412286
- PMCID: PMC3131697
- DOI: 10.1038/gt.2011.3
AAV-mediated photoreceptor transduction of the pig cone-enriched retina
Abstract
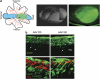
Recent success in clinical trials supports the use of adeno-associated viral (AAV) vectors for gene therapy of retinal diseases caused by defects in the retinal pigment epithelium (RPE). In contrast, evidence of the efficacy of AAV-mediated gene transfer to retinal photoreceptors, the major site of inherited retinal diseases, is less robust. In addition, although AAV-mediated RPE transduction appears efficient, independently of the serotype used and species treated, AAV-mediated photoreceptor gene transfer has not been systematically investigated thus so far in large animal models, which also may allow identifying relevant species-specific differences in AAV-mediated retinal transduction. In the present study, we used the porcine retina, which has a high cone/rod ratio. This feature allows to properly evaluate both cone and rod photoreceptors transduction and compare the transduction characteristics of AAV2/5 and 2/8, the two most efficient AAV vector serotypes for photoreceptor targeting. Here we show that AAV2/5 and 2/8 transduces both RPE and photoreceptors. AAV2/8 infects and transduces photoreceptor more efficiently than AAV2/5, similarly to what we have observed in the murine retina. The use of the photoreceptor-specific rhodopsin promoter restricts transgene expression to porcine rods and cones, and results in photoreceptor transduction levels similar to those obtained with the ubiquitous promoters tested. Finally, immunological, toxicological and biodistribution studies support the safety of AAV subretinal administration to the large porcine retina. The data presented here on AAV-mediated transduction of the cone-enriched porcine retina may affect the development of gene-based therapies for rare and common severe photoreceptor diseases.
Figures



References
-
- Cremers FP, van den Hurk JA, den Hollander AI. Molecular genetics of Leber congenital amaurosis. Hum Mol Genet. 2002;11:1169–1176. - PubMed
-
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

