Residual beta cell function in newly diagnosed type 1 diabetes after treatment with atorvastatin: the Randomized DIATOR Trial
- PMID: 21412424
- PMCID: PMC3055882
- DOI: 10.1371/journal.pone.0017554
Residual beta cell function in newly diagnosed type 1 diabetes after treatment with atorvastatin: the Randomized DIATOR Trial
Abstract
Background: Recent evidence suggests that the lipid-lowering agent atorvastatin is also a potent immunomodulator. The aim of this study was to investigate the possible effect of atorvastatin on the decline of residual beta cell function in recent-onset type 1 diabetes.
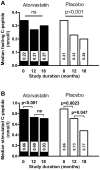
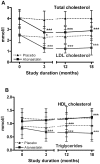
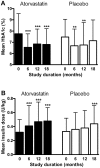
Methods and findings: The randomised placebo-controlled Diabetes and Atorvastatin (DIATOR) Trial included 89 patients with newly diagnosed type 1 diabetes and islet autoantibodies (mean age 30 years, 40% females), in 12 centres in Germany. Patients received placebo or 80 mg/d atorvastatin for 18 months. As primary outcome stimulated serum C-peptide levels were determined 90 min after a standardized liquid mixed meal. An intent-to-treat analysis was performed. Fasting and stimulated C-peptide levels were not significantly different between groups at 18 months. However, median fasting serum C-peptide levels dropped from baseline to 12 and 18 months in the placebo group (from 0. 34 to 0.23 and 0.20 nmol/l, p<0.001) versus a nonsignificant decline in the atorvastatin group (from 0.34 to 0.27 and 0.30 nmol/l, ns). Median stimulated C-peptide concentrations declined between baseline and 12 months (placebo from 0.89 to 0.71 nmol/l, atorvastatin from 0.88 to 0.73 nmol/l, p<0.01 each) followed by a major loss by month 18 in the placebo group (to 0.48 nmol/l, p = 0.047) but not in the atorvastatin group (to 0.71 nmol/l, ns). Median levels of total cholesterol and C-reactive protein decreased in the atorvastatin group only (p<0.001 and p = 0.04). Metabolic control was similar between groups.
Conclusions: Atorvastatin treatment did not significantly preserve beta cell function although there may have been a slower decline of beta-cell function which merits further study.
Trial registration: ClinicalTrials.gov NCT00974740.
Conflict of interest statement
Figures

References
-
- The Canandian/European Randomized Control Trial Group. Cyclosporin-induced remission of IDDM after early intervention. Association of 1 yr of cyclosporin treatment with enhanced insulin secretion. The Canadian-European Randomized Control Trial Group. Diabetes. 1988;37:1574–1582. - PubMed
-
- Waldron-Lynch F, Herold KC. Advances in Type 1 diabetes therapeutics: immunomodulation and beta-cell salvage. Endocrinol Metab Clin North Am. 2009;38:303–17, viii. - PubMed
-
- Blank N, Schiller M, Krienke S, Busse F, Schatz B, et al. Atorvastatin inhibits T cell activation through 3-hydroxy-3-methylglutaryl coenzyme A reductase without decreasing cholesterol synthesis. J Immunol. 2007;179:3613–3621. - PubMed
-
- Bonnet J, McPherson R, Tedgui A, Simoneau D, Nozza A, et al. Comparative effects of 10-mg versus 80-mg Atorvastatin on high-sensitivity C-reactive protein in patients with stable coronary artery disease: results of the CAP (Comparative Atorvastatin Pleiotropic effects) study. Clin Ther. 2008;30:2298–2313. - PubMed
-
- Weitz-Schmidt G, Welzenbach K, Brinkmann V, Karnata T, Kallen J, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–692. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

