A live-attenuated HSV-2 ICP0 virus elicits 10 to 100 times greater protection against genital herpes than a glycoprotein D subunit vaccine
- PMID: 21412438
- PMCID: PMC3055896
- DOI: 10.1371/journal.pone.0017748
A live-attenuated HSV-2 ICP0 virus elicits 10 to 100 times greater protection against genital herpes than a glycoprotein D subunit vaccine
Abstract
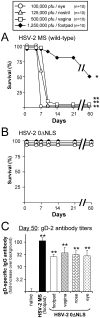
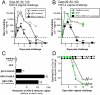
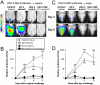
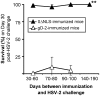
Glycoprotein D (gD-2) is the entry receptor of herpes simplex virus 2 (HSV-2), and is the immunogen in the pharmaceutical industry's lead HSV-2 vaccine candidate. Efforts to prevent genital herpes using gD-2 subunit vaccines have been ongoing for 20 years at a cost in excess of $100 million. To date, gD-2 vaccines have yielded equivocal protection in clinical trials. Therefore, using a small animal model, we sought to determine if a live-attenuated HSV-2 ICP0⁻ virus would elicit better protection against genital herpes than a gD-2 subunit vaccine. Mice immunized with gD-2 and a potent adjuvant (alum+monophosphoryl lipid A) produced high titers of gD-2 antibody. While gD-2-immunized mice possessed significant resistance to HSV-2, only 3 of 45 gD-2-immunized mice survived an overwhelming challenge of the vagina or eyes with wild-type HSV-2 (MS strain). In contrast, 114 of 115 mice immunized with a live HSV-2 ICP0⁻ virus, 0ΔNLS, survived the same HSV-2 MS challenges. Likewise, 0ΔNLS-immunized mice shed an average 125-fold less HSV-2 MS challenge virus per vagina relative to gD-2-immunized mice. In vivo imaging demonstrated that a luciferase-expressing HSV-2 challenge virus failed to establish a detectable infection in 0ΔNLS-immunized mice, whereas the same virus readily infected naïve and gD-2-immunized mice. Collectively, these results suggest that a HSV-2 vaccine might be more likely to prevent genital herpes if it contained a live-attenuated HSV-2 virus rather than a single HSV-2 protein.
Conflict of interest statement
Figures




Similar articles
-
Antibodies Are Required for Complete Vaccine-Induced Protection against Herpes Simplex Virus 2.PLoS One. 2015 Dec 15;10(12):e0145228. doi: 10.1371/journal.pone.0145228. eCollection 2015. PLoS One. 2015. PMID: 26670699 Free PMC article.
-
Herpes simplex virus 2 (HSV-2) infected cell proteins are among the most dominant antigens of a live-attenuated HSV-2 vaccine.PLoS One. 2015 Feb 6;10(2):e0116091. doi: 10.1371/journal.pone.0116091. eCollection 2015. PLoS One. 2015. PMID: 25658852 Free PMC article.
-
Immunogenicity and efficacy of intramuscular replication-defective and subunit vaccines against herpes simplex virus type 2 in the mouse genital model.PLoS One. 2012;7(10):e46714. doi: 10.1371/journal.pone.0046714. Epub 2012 Oct 11. PLoS One. 2012. PMID: 23071620 Free PMC article.
-
The challenge of developing a herpes simplex virus 2 vaccine.Expert Rev Vaccines. 2012 Dec;11(12):1429-40. doi: 10.1586/erv.12.129. Expert Rev Vaccines. 2012. PMID: 23252387 Free PMC article. Review.
-
Antigenic breadth: a missing ingredient in HSV-2 subunit vaccines?Expert Rev Vaccines. 2014 Jun;13(6):691-710. doi: 10.1586/14760584.2014.910121. Expert Rev Vaccines. 2014. PMID: 24837838 Review.
Cited by
-
Griffithsin protects mice from genital herpes by preventing cell-to-cell spread.J Virol. 2013 Jun;87(11):6257-69. doi: 10.1128/JVI.00012-13. Epub 2013 Mar 27. J Virol. 2013. PMID: 23536670 Free PMC article.
-
An Insight into Current Treatment Strategies, Their Limitations, and Ongoing Developments in Vaccine Technologies against Herpes Simplex Infections.Vaccines (Basel). 2023 Jan 17;11(2):206. doi: 10.3390/vaccines11020206. Vaccines (Basel). 2023. PMID: 36851084 Free PMC article. Review.
-
HSV-2 vaccine: current status and insight into factors for developing an efficient vaccine.Viruses. 2014 Jan 24;6(2):371-90. doi: 10.3390/v6020371. Viruses. 2014. PMID: 24469503 Free PMC article. Review.
-
A herpes simplex virus 2 (HSV-2) glycoprotein D-expressing nonreplicating dominant-negative HSV-2 virus vaccine is superior to a gD2 subunit vaccine against HSV-2 genital infection in guinea pigs.PLoS One. 2014 Jun 30;9(6):e101373. doi: 10.1371/journal.pone.0101373. eCollection 2014. PLoS One. 2014. PMID: 24979708 Free PMC article.
-
Toward the Eradication of Herpes Simplex Virus: Vaccination and Beyond.Viruses. 2024 Sep 17;16(9):1476. doi: 10.3390/v16091476. Viruses. 2024. PMID: 39339952 Free PMC article. Review.
References
-
- CDC. Seroprevalence of herpes simplex virus type 2 among persons aged 14–49 years–United States, 2005–2008. MMWR Morb Mortal Wkly Rep. 2010;59:456–459. - PubMed
-
- Gottlieb SL, Douglas JM, Jr, Foster M, Schmid DS, Newman DR, et al. Incidence of herpes simplex virus type 2 infection in 5 sexually transmitted disease (STD) clinics and the effect of HIV/STD risk-reduction counseling. J Infect Dis. 2004;190:1059–1067. - PubMed
-
- Jonsson MK, Wahren B. Sexually transmitted herpes simplex viruses. Scand J Infect Dis. 2004;36:93–101. - PubMed
-
- Bernstein DI. Potential for immunotherapy in the treatment of herpesvirus infections. Herpes. 2001;8:8–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

