Cannabinoid type 1 receptor antagonists for smoking cessation
- PMID: 21412887
- PMCID: PMC6486173
- DOI: 10.1002/14651858.CD005353.pub4
Cannabinoid type 1 receptor antagonists for smoking cessation
Abstract
Background: Selective type 1 cannabinoid (CB1) receptor antagonists may assist with smoking cessation by restoring the balance of the endocannabinoid system, which can be disrupted by prolonged use of nicotine. They also seeks to address many smokers' reluctance to persist with a quit attempt because of concerns about weight gain.
Objectives: To determine whether selective CB1 receptor antagonists (currently rimonabant and taranabant) increase the numbers of people stopping smoking To assess their effects on weight change in successful quitters and in those who try to quit but fail.
Search strategy: We searched the Cochrane Tobacco Addiction Review Group specialized register for trials, using the terms ('rimonabant' or 'taranabant') and 'smoking' in the title or abstract, or as keywords. We also searched MEDLINE, EMBASE, CINAHL and PsycINFO, using major MESH terms. We acquired electronic or paper copies of posters of preliminary trial results presented at the American Thoracic Society Meeting in 2005, and at the Society for Research on Nicotine and Tobacco European Meeting 2006. We also attempted to contact the authors of ongoing studies of rimonabant, and Sanofi Aventis (manufacturers of rimonabant). The most recent search was in January 2011.
Selection criteria: Types of studies Randomized controlled trialsTypes of participants Adult smokersTypes of interventions Selective CB1 receptor antagonists, such as rimonabant and taranabant. Types of outcome measures The primary outcome is smoking status at a minimum of six months after the start of treatment. We preferred sustained cessation rates to point prevalence, and biochemically verified cessation to self-reported quitting. We regarded smokers who drop out or are lost to follow up as continuing smokers. We have noted any adverse effects of treatment.A secondary outcome is weight change associated with the cessation attempt.
Data collection and analysis: Two authors checked the abstracts for relevance, and attempted to acquire full trial reports. One author extracted the data, and a second author checked them.
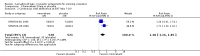
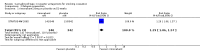
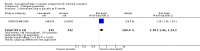
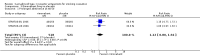
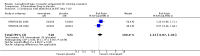
Main results: We found three trials which met our inclusion criteria, covering 1567 smokers (cessation: STRATUS-EU and STRATUS-US), and 1661 quitters (relapse prevention: STRATUS-WW). At one year, the pooled risk ratio (RR) for quitting with rimonabant 20 mg was 1.50 (95% confidence interval (CI) 1.10 to 2.05). No significant benefit was demonstrated for rimonabant at 5 mg dosage. Adverse events included nausea and upper respiratory tract infections. In the relapse prevention trial, smokers who had quit on the 20 mg regimen were more likely to remain abstinent on either active regimen than on placebo; the RR for the 20 mg maintenance group was 1.29 (95% CI 1.06 to 1.57), and for the 5 mg maintenance group 1.30 (95% CI 1.06 to 1.59). There appeared to be no significant benefit of maintenance treatment for the 5 mg quitters. One trial of taranabant was not included in our meta-analyses, as it followed participants only until end of treatment; at eight weeks it found no benefit for treatment over placebo, with an OR of 1.2 (90% CI 0.6 to 2.5). For rimonabant, weight gain was reported to be significantly lower among the 20 mg quitters than in the 5 mg or placebo quitters. During treatment, overweight or obese smokers tended to lose weight, while normal weight smokers did not. For taranabant, weight gain was significantly lower for 2-8 mg versus placebo at the end of eight weeks of treatment. In 2008, post-marketing surveillance led the European Medicines Agency (EMEA) to require Sanofi Aventis to withdraw rimonabant, because of links to mental disorders. The development of taranabant was also suspended by Merck & Co because of unacceptable adverse events.
Authors' conclusions: From the trial reports available, rimonabant 20 mg may increase the chances of quitting approximately 1½-fold. The evidence for rimonabant in maintaining abstinence is inconclusive. Rimonabant 20 mg may moderate weight gain in the long term. Taranabant 2-8 mg may moderate weight gain, at least in the short term. In 2008, development of both rimonabant and taranabant was discontinued by the manufacturers.
Conflict of interest statement
None known
Figures



Update of
-
Cannabinoid type 1 receptor antagonists (rimonabant) for smoking cessation.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD005353. doi: 10.1002/14651858.CD005353.pub3. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2011 Mar 16;(3):CD005353. doi: 10.1002/14651858.CD005353.pub4. PMID: 17943852 Updated.
References
References to studies included in this review
STRATUS‐EU 2006 {published and unpublished data}
-
- Cinciripini PM, Aubin H‐J, Dale LC, Niaura R, Anthenelli RM, Robinson J. Pooled analysis of three short‐term, randomised, double‐blind, placebo‐controlled trials with rimonabant 20 mg/d in smoking cessation [PI‐TS‐01]. Society for Research on Nicotine and Tobacco 8th European Conference, September 2006, Kusadasi Turkey. 2006. [clinicaltrials.gov ID NCT00464165]
-
- Sanofi Aventis. Information Meeting. http://en.sanofi‐aventis.com/Images/en_050301_up_2004_Full_Year_Results_Analysts_Investor... (accessed 23rd November 2006) 2005.
STRATUS‐US 2006 {published and unpublished data}
-
- Anthenelli RM, Depres J‐P. Effects of rimonabant in the reduction of major cardiovascular risk factors. Results from the STRATUS‐US Trial (smoking cessation in smokers motivated to quit) and the RIO‐LIPIDS Trial (weight reducing and metabolic effects in overweight/obese patients with dyslipidemia. American College of Cardiology Annual Scientific Session. March 2004.
-
- Boyd ST, Fremming BA. Rimonabant ‐ a selective CB1 antagonist. Annals of Pharmacotherapy 2005;39:684‐90. - PubMed
-
- Cinciripini PM, Aubin H‐J, Dale LC, Niaura R, Anthenelli RM, Robinson J. Pooled analysis of three short‐term, randomised, double‐blind, placebo‐controlled trials with rimonabant 20 mg/d in smoking cessation [PI‐TS‐01]. Society for Research on Nicotine and Tobacco 8th European Conference, September 2006, Kusadasi Turkey. 2006. [clinicaltrials.gov ID NCT00358228]
STRATUS‐WW 2005 {published and unpublished data}
-
- Niaura R. Long‐term maintenance of abstinence from smoking with rimonabant: results from the STRATUS Worldwide trial. 1‐year efficacy/safety results. American Thoracic Society Conference 2005. [clinical trials.gov ID NCT00459173]
-
- Niaura R. Long‐term maintenance of abstinence from smoking with rimonabant: results from the STRATUS Worldwide trial. 6‐month efficacy/safety results [POS1‐054]. American Thoracic Society Conference. 2004.
-
- Sanofi Aventis. Information Meeting. http://en.sanofi‐aventis.com/Images/en_050301_up_2004_Full_Year_Results_Analysts_Investor... (accessed 23rd November 2006) 2005.
References to studies excluded from this review
Morrison 2010 {published data only}
-
- Morrison MF, Ceesay P, Gantz I, Kaufman KD, Lines CR. Randomized, controlled, double‐blind trial of taranabant for smoking cessation. Psychopharmacology 2010;209:245‐53. [clinicaltrials.gov ID NCT00109135] - PubMed
Rigotti 2009 {published data only}
-
- Rigotti NA, Gonzales D, Dale LC, Lawrence D, Chang Y. A randomized controlled trial of adding the nicotine patch to rimonabant for smoking cessation: efficacy, safety and weight gain. Addiction 2009;104(2):266‐76. - PubMed
-
- Stapleton J. Trial comes too late as psychiatric side effects end hope for rimonabant. Addiction 2009;104(2):277‐8. - PubMed
STRATUS META 2006 {unpublished data only}
-
- Cinciripini PM, Aubin H‐J, Dale LC, Niaura R, Anthenelli RM, Robinson J. Pooled analysis of three short‐term, randomised, double‐blind, placebo‐controlled trials with rimonabant 20 mg/d in smoking cessation [PI‐TS‐01]. Society for Research on Nicotine and Tobacco 8th European Conference, September 2006, Kusadasi Turkey. 2006.
-
- Sanofi Aventis. Comparison of efficacy and safety of rimonabant 20mg/day versus placebo in smoking cessation. http://clinicaltrials.gov/show/NCT00464256 (accessed 17th October 2007) 2007. [clinicaltrials.gov ID NCT00464256]
Topol 2010 {published data only}
-
- Topol EJ, Bousser MG, Fox KA, Creager MA, Despres JP, Easton JD, et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo‐controlled trial. Lancet 2010;376(9740):517‐23. [clinicaltrials.gov ID NCT00263042] - PubMed
Additional references
ACC 2004
-
- American College of Cardiology. Data shows new drug, rimonabant, helps smokers quit while limiting post cessation weight gain. www.eurekalert.org/pub_releases/2004‐03/k‐dsn030904.php (accessed September 15 2004).
Acomplia SPC 2006
-
- Sanofi‐Aventis. Acomplia 20mg Summary of Product Characteristics. http://emc.medicines.org.uk/emc/assets/c/html/displaydoc.asp?documentid=... (accessed 18th April 2007) 2006.
Aronne 2010
-
- Aronne LJ, Tonstad S, Moreno M, Gantz I, Erondu N, Suryawanshi S, et al. A clinical trial assessing the safety and efficacy of taranabant, a CB1R inverse agonist, in obese and overweight patients: a high‐dose study. International Journal of Obesity 2010;34(5):919‐35. - PubMed
Cleland 2004
-
- Cleland JGF, Ghosh J, Freemantle N, Kaye GC, Nasir M, Clark AL, et al. Clinical trials update and cumulative meta‐analyses from the American College of Cardiology: WATCH, SCD‐HeFT, DINAMIT, CASINO, INSPIRE, STRATUS‐US, RIO‐LIPIDS and cardiac resynchronisation therapy in heart failure. European Journal of Heart Failure 2004;6:501‐8. - PubMed
Cochrane Handbook
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. http://www.cochrane.org/resources/handbook/hbook.htm (accessed 18th April 2007) 2006.
Curioni 2006
Fagerström 2006
-
- Fagerström K, Balfour DJK. Neuropharmacology and potential efficacy of new treatments for tobacco dependence. Expert Opinion on Investigational Drugs 2006;15(2):107‐16. - PubMed
FDA 2007
-
- Food, Drug Administration. Summary Minutes of the Endocrinologic and Metabolic Drugs Advisory Committee meeting on June 13, 2007. www.fda.gov/ohrms/dockets/ac/07/minutes/2007‐4306m1‐final.pdf (accessed 6th August 2007) 2007.
Filozof 2004
-
- Filozof C, Pinilla MC, Fernandez‐Cruz A. Smoking cessation and weight gain. Obesity Review 2004;5:95‐103. - PubMed
Fremming 2008
-
- Fremming BA, Boyd ST. Taranabant, a novel cannabinoid type 1 receptor inverse agonist. Current Opinion in Investigational Drugs 2008;9(10):1116‐29. - PubMed
Gelfand 2006
-
- Gelfand EV, Cannon CP. Rimonabant: a selective blocker of the cannabinoid CB1 receptors for the management of obesity, smoking cessation and cardiometabolic risk factors. Expert Opinion on Investigational Drugs 2006;15(3):307‐15. - PubMed
Hughes 2003
-
- Hughes JR, Keely JP, Niaura RS, Ossip‐Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research 2003;5(1):13‐25. - PubMed
Le Foll 2008
Mizoue 1998
-
- Mizoue T, Ueda R, Tokui N, Hino Y, Yoshimura T. Body mass decrease after initial gain following smoking cessation. International Journal of Epidemiology 1998;27:984‐8. - PubMed
O'Hara 1998
-
- O'Hara P, Connett JE, Lee WW, Nides M, Murray R, Wise R. Early and late weight gain following smoking cessation in the Lung Health Study. American Journal of Epidemiology 1998;148(9):821‐30. - PubMed
ONS 2002
-
- Office for National Statistics. Smoking‐related behaviour and attitudes. http://www.dh.gov.uk/assetRoot/04/06/81/56/04068156.pdf (accessed 11th April 2005).
Pomerleau 2000
-
- Pomerleau CS, Pomerleau OF, Namenek RJ, Mehringer AM. Short‐term weight gain in abstaining women smokers. Journal of Substance Abuse and Treatment 2000;18(4):339‐42. - PubMed
Sanofi 2003
-
- Sanofi‐‐Synthelabo. RIO and STRATUS Clinical Studies Guide. Sanofi‐Synthelabo, 2003.
Sanofi Aventis 2008
-
- Sanofi Aventis. Acomplia update post EMEA recommendation. http://en.sanofi‐aventis.com/binaries/20081023_Acomplia_Update_presentation_tcm28‐22428.pdf 2008.
Sanofi‐Synth 2004
-
- Sanofi‐Synthelabo. Two pivotal studies indicate ACOMPLIA (rimonabant) offers a novel approach to cardiovascular risk management in overweight/obese people and smokers. http://es.sanofi‐synthelabo.com/press/ppc_23804.asp (accessed September 15 2004).
USPHS 1990
-
- US Public Health Service. The health benefits of smoking cessation: A report of the Surgeon General. DHHS Publication No CDC 90‐8416 1990; Vol. Washington DC.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

