Celiac plexus block for pancreatic cancer pain in adults
- PMID: 21412903
- PMCID: PMC6464722
- DOI: 10.1002/14651858.CD007519.pub2
Celiac plexus block for pancreatic cancer pain in adults
Abstract
Background: Pancreatic cancer causes severe pain in 50 to 70% of patients and is often difficult to treat. Celiac plexus block (CPB) is thought to be a safe and effective technique for reducing the severity of pain.
Objectives: To determine the efficacy and safety of celiac plexus neurolysis in reducing pancreatic cancer pain, and to identify adverse effects and differences in efficacy between the different techniques.
Search strategy: We searched Cochrane CENTRAL, MEDLINE, GATEWAY and EMBASE from 1990 to December 2010.
Selection criteria: Randomised controlled trials (RCTs) of CPB by the percutaneous approach or endoscopic ultrasonography (EUS)-guided neurolysis in adults with pancreatic cancer at any stage, with a minimum of four weeks follow-up.
Data collection and analysis: We recorded details of study design, participants, disease, setting, outcome assessors, pain intensity (visual analogue scale (VAS)) and methods of calculation.
Main results: The search identified 102 potentially eligible studies. Judged from the information in the title and abstract six of these concerning the percutaneous block, involving 358 participants, fulfilled the inclusion criteria and were included in the review. All were RCTs in which the participants were followed for at least four weeks. We excluded studies published only as abstracts. We identified one RCT comparing EUS-guided or computed tomography (CT) -guided CPB but its aim was to assess efficacy in controlling chronic abdominal pain associated with chronic pancreatitis rather than pancreatic cancer, so it was excluded.For pain (VAS) at four weeks the mean difference was -0.42 in favour of CPB (95% confidence interval (CI) -0.70 to - 0.13, P = 0.004, fixed-effect model). At eight weeks the mean difference was -0.44 (95% CI -0.89 to - 0.01, random-effects model). At eight weeks there was significant heterogeneity (I(2) = 89%).Opioid consumption was significantly lower in the CPB group than the control group (P < 0.00001).
Authors' conclusions: Although statistical evidence is minimal for the superiority of pain relief over analgesic therapy, the fact that CPB causes fewer adverse effects than opioids is important for patients. Further studies and RCTs are recommended to demonstrate the potential efficacy of a less invasive technique under EUS guidance.
Conflict of interest statement
None known
Figures
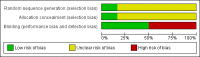



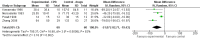







Update of
- doi: 10.1002/14651858.CD007519
Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Hydromorphone for cancer pain.Cochrane Database Syst Rev. 2016 Oct 11;10(10):CD011108. doi: 10.1002/14651858.CD011108.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2021 Aug 5;8:CD011108. doi: 10.1002/14651858.CD011108.pub3. PMID: 27727452 Free PMC article. Updated.
-
Oxycodone for cancer-related pain.Cochrane Database Syst Rev. 2017 Aug 22;8(8):CD003870. doi: 10.1002/14651858.CD003870.pub6. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Jun 9;6:CD003870. doi: 10.1002/14651858.CD003870.pub7. PMID: 28829910 Free PMC article. Updated.
Cited by
-
Efficacy of splanchnic nerve neurolysis in the management of upper abdominal cancer pain: A systematic review and meta-analysis.Indian J Anaesth. 2023 Dec;67(12):1036-1050. doi: 10.4103/ija.ija_439_23. Epub 2023 Dec 13. Indian J Anaesth. 2023. PMID: 38343676 Free PMC article.
-
Impact of celiac neurolysis on survival in patients with pancreatic cancer.Gastrointest Endosc. 2015 Jul;82(1):46-56.e2. doi: 10.1016/j.gie.2014.12.036. Epub 2015 Mar 20. Gastrointest Endosc. 2015. PMID: 25800661 Free PMC article.
-
Current role of endoscopic ultrasound in the diagnosis and management of pancreatic cancer.World J Gastrointest Endosc. 2022 Jan 16;14(1):35-48. doi: 10.4253/wjge.v14.i1.35. World J Gastrointest Endosc. 2022. PMID: 35116098 Free PMC article. Review.
-
Role of endoscopic ultrasound in anticancer therapy: Current evidence and future perspectives.World J Gastrointest Oncol. 2021 Dec 15;13(12):1863-1879. doi: 10.4251/wjgo.v13.i12.1863. World J Gastrointest Oncol. 2021. PMID: 35070030 Free PMC article. Review.
-
Role of endoscopic ultrasound in the diagnosis of pancreatic cancer.World J Gastrointest Oncol. 2014 Sep 15;6(9):360-8. doi: 10.4251/wjgo.v6.i9.360. World J Gastrointest Oncol. 2014. PMID: 25232461 Free PMC article. Review.
References
References to studies included in this review
Kawamata 1996 {published data only}
-
- Kawamata M, Ishitani K, Ishikawa K, Sasaki H, Ota K, Omote K, et al. Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer pain. Pain 1996;64(3):597‐602. - PubMed
Lillemoe 1993 {published data only}
Mercadante 1993 {published data only}
-
- Mercadante S. Celiac plexus block versus analgesics in pancreatic cancer pain. Pain 1993;52(2):187‐92. - PubMed
Polati 1998 {published data only}
-
- Polati E, Finco G, Gottin L, Bassi C, Pederzoli P, Ischia S. Prospective randomized double‐blind trial of neurolytic coeliac plexus block in patients with pancreatic cancer. British Journal of Surgery 1998;85(2):199‐201. - PubMed
Wong 2004 {published data only}
-
- Wong GY, Schroeder DR, Carns PE, Wilson JL, Martin DP, Kinney MO, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA 2004;291(9):1092‐9. - PubMed
Zhang 2008 {published data only}
-
- Zhang CL, Zhang TJ, Guo YN, Yang LQ, He MW, Shi JZ, et al. Effect of neurolytic celiac plexus block guided by computerized tomography on pancreatic cancer pain. Digestive Diseases Sciences 2008;53(3):856‐60. - PubMed
References to studies excluded from this review
Gress 1999 {published data only}
-
- Gress F, Schmitt C, Sherman S, Ikenberry S, Lehman G. A prospective randomized comparison of endoscopic ultrasound‐ and computed tomography‐guided celiac plexus block for managing chronic pancreatitis pain. American Journal of Gastroenterology 1999;94(4):872‐4. - PubMed
Gunaratnam 2001 {published data only}
-
- Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS‐guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointestinal Endoscopy 2001;54(3):316‐24. - PubMed
Levy 2008 {published data only}
-
- Levy MJ, Topazian MD, Wiersema MJ, Clain JE, Rajan E, Wang KK, et al. Initial evaluation of the efficacy and safety of endoscopic ultrasound‐guided direct ganglia neurolysis and block. American Journal of Gastroenterology 2008;103(1):98‐103. - PubMed
Sakamoto 2010 {published data only}
-
- Sakamoto H, Kitano M, Kamata K, Komaki T, ImaiH, Chikugo T, et al. EUS‐guided broad plexus neurolysis over the superior mesenteric artery using a 25‐gauge needle. American Journal of Gastroenterology 2010;105(12):2599‐606. - PubMed
Tran 2006 {published data only}
-
- Tran QN, Urayama S, Meyers FJ. Endoscopic ultrasound‐guided celiac plexus neurolysis for pancreatic cancer pain: a single institution experience and review of the literature. Journal of Supportive Oncology 2006;4(9):460‐2. - PubMed
Wiersema 1996 {published data only}
-
- Wiersema MJ, Wiersema LM. Endosonography‐guided celiac plexus neurolysis. Gastrointestinal Endoscopy. 1996;44(6):656‐62. - PubMed
Additional references
Giménez 1993
-
- Giménez A, Martinez‐Noguera A, Donoso L, Català E, Serra R. Percutaneous neurolysis of the celiac plexus via the anterior approach with sonographic guidance. American Journal of Roentgenology 1993;161(5):1061‐3. - PubMed
Higgins 2008
-
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. The Cochrane Collaboration 2008. Available from www.cochrane‐handbook.org, updated February 2008.
Kappis 1919
-
- Kappis M. Sensitivity (means "pain sensation" in this context) and local anesthesia in the surgical area of the abdominal cavity, with special focus on anesthesia of the splanchnic nerve [Sensibilitat und lokale Anasthesie im chirurgischen Gebiet der Bauchhohle mit besonderer Berucksichtigung der splanchnicus‐Aasthesie]. Beitrage zur klinischen chirurgie 1919;115:161‐75.
Maisonneuve 2010
-
- Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis. 2010;28(4‐5):645‐56. - PubMed
Michaels 2007
Moore 1981
-
- Moore DC, Busch WH, Burnett LL. Celiac plexus block: a roentgenographic, anatomic study of technique and spread of solution in patients and corpses. Anesthesia and Analgesia 1981;60:369‐79. - PubMed
Puli 2009
-
- Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS‐guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta‐analysis and systematic review. Digestive Diseases Science 2009;54(11):2330‐7. - PubMed
Staatas 2001
-
- Staatas PS, Hekmat H, Sauter P, Lillemoe K. The effects of alcohol celiac block, pain, and mood on longevity in patients with unresectable pancreatic pain: a double blind, randomised, placebo‐controlled study. Pain Medicine 2001;2(1):28‐34. - PubMed
WHO 2008
-
- Scoping Document for WHO Treatment Guideline on Pain Related to Cancer, HIV and other progressive life‐threatening illnesses in adults Adopted in WHO Steering Group on Pain Guidelines, 14 October 2008. WHO Steering Group on Pain Guidelines.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

