If C-H bonds could talk: selective C-H bond oxidation
- PMID: 21413105
- PMCID: PMC3980681
- DOI: 10.1002/anie.201006368
If C-H bonds could talk: selective C-H bond oxidation
Abstract
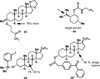
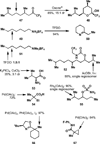
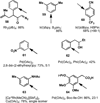
C-H oxidation has a long history and an ongoing presence in research at the forefront of chemistry and interrelated fields. As such, numerous highly useful articles and reviews have been written on this subject. Logically, these are generally written from the perspective of the scope and limitations of the reagents employed. This Minireview instead attempts to emphasize chemoselectivity imposed by the nature of the substrate. Consequently, many landmark discoveries in the field of C-H oxidation are not discussed, but hopefully the perspective taken herein will allow C-H oxidation reactions to be more readily incorporated into synthetic planning.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


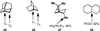
References
-
-
For representative examples and the importance of C–H activation in chemical synthesis, see: Gaich T, Baran PS. J. Org. Chem. 2010;75:4657–4673.
-
-
-
For an overview with respect to taxol, see: Ishihara Y, Baran PS. Synlett. 2010;12:1733–1745.
-
-
-
For reviews on C–H oxidation, see: Barton DHR, Doller D. Acc. Chem. Res. 1992;25:504–512. Barton DHR. Tetrahedron. 1998;54:5805–5817. Doyle MP, Forbes DC. Chem. Rev. 1998;98:911–935. Müller P, Fruit C. Chem. Rev. 2003;103:2905–2919. Davies HML, Beckwith REJ. Chem. Rev. 2003;103:2861–2904. Punniyamurthy T, Velusamy S, Iqbal J. Chem. Rev. 2005;105:2329–2363. Godula K, Sames D. Science. 2006;312:67–72. Dick AR, Sanford MS. Tetrahedron. 2006;62:2439–2463. Davies HML, Manning JR. Nature. 2008;451:417–424. Giri R, Shi B-F, Engle KM, Maugel N, Yu J-Q. Chem. Soc. Rev. 2009;38:3242–3271. Doyle MP, Duffy R, Ratnikov M, Zhou L. Chem. Rev. 2010;110:704–724.
-
-
-
For reviews on C–H oxidation from an organometallic perspective, see: Arndtsen BA, Bergman RG, Mobley TA, Peterson TH. Acc. Chem. Res. 1995;28:154–162. Shilov AE, Shul’pin GB. Chem. Rev. 1997;97:2879–2932. Stahl SS, Labinger JA, Bercaw JE. Angew. Chem. Int. Ed. 1998;37:2180. Jones WD. Top. Organomet. Chem. 1999;3:9–46. Crabtree RH. J. Chem. Soc., Dalton Trans. 2001:2437. Pamplin CB, Legzdins P. Acc. Chem. Res. 2003;36:223–233. Lersch M, Tilset M. Chem. Rev. 2005;105:2471–2526. Campeau L-C, Stuart DR, Fagnou K. Aldrichimica Acta. 2007;40:35–41. Kulkarni AA, Daugulis O. Synthesis. 2009:4087–4109. Daugulis O, Do H-Q, Shabashov D. Acc. Chem. Res. 2009;42:1074–1086. Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem. Rev. 2010;110:890–931. Shul’pin GB. Org. Biomol. Chem. 2010;8:4217–4228. Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem. Eur. J. 2010;16:2654–2672. Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147–1169. Yu J-Q, Shi Z, editors. C–H Activation, Top. Curr. Chem. 2010:292.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

