Type I collagen, fibrin and PuraMatrix matrices provide permissive environments for human endothelial and mesenchymal progenitor cells to form neovascular networks
- PMID: 21413157
- PMCID: PMC3178449
- DOI: 10.1002/term.389
Type I collagen, fibrin and PuraMatrix matrices provide permissive environments for human endothelial and mesenchymal progenitor cells to form neovascular networks
Abstract
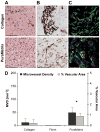
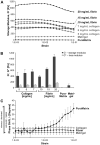
The field of tissue engineering seeks to create metabolically demanding, functional tissues, which will require blood vessel networks capable of forming rapidly in a variety of extracellular matrix (ECM) environments. We tested whether human endothelial progenitor cells (EPCs) and mesenchymal progenitor cells (MPCs) could form microvascular networks in type I collagen, fibrin and an engineered peptide hydrogel, PuraMatrix, in 7 days in vivo in immune-deficient mice. These results are compared to those previously published, based on the Matrigel ECM. Perfused blood vessels formed in all three types of ECM within 7 days. Collagen at 5 and 6 mg/ml and 10 mg/ml fibrin supported vessel formation at 30-60 vessels/mm(2), and PuraMatrix enabled vessel formation to 160 vessels/mm(2), significantly greater than collagen or fibrin. Vessels were composed of EPCs with perivascular cells on their abluminal surfaces. EPCs injected alone formed a low density of blood vessels in collagen and PuraMatrix, while MPCs injected alone resulted in sparse vessel networks in all ECMs tested. A rheometer was used to determine whether the ECMs which supported vascularization had bulk physical properties similar to or distinct from Matrigel. Collagen and fibrin were the stiffest matrices to support extensive vascularization, with storage moduli in the range 385-510 Pa, while Matrigel, at 80 Pa, and PuraMatrix, at 5 Pa, were far more compliant. Thus, EPCs and MPCs were capable of vasculogenesis in environments having disparate physical properties, although vascular density was greater in more compliant ECMs. We propose that EPC/MPC-mediated vascularization is a versatile technology which may enable the development of engineered organs.
Copyright © 2011 John Wiley & Sons, Ltd.
Conflict of interest statement
The authors indicate that no competing financial interests exist.
Figures


References
-
- Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821–823. - PubMed
-
- Egana JT, Fierro FA, Kruger S, Bornhauser M, Huss R, Lavandero S, Machens HG. Use of human mesenchymal cells to improve vascularization in a mouse model for scaffold-based dermal regeneration. Tissue Eng Part A. 2009;15:1191–1200. - PubMed
-
- Cabodi M, Choi NW, Gleghorn JP, Lee CS, Bonassar LJ, Stroock AD. A microfluidic biomaterial. J Am Chem Soc. 2005;127:13788–13789. - PubMed
-
- Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720–725. - PubMed
-
- Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

