Circadian disruption and SCN control of energy metabolism
- PMID: 21414317
- PMCID: PMC3095769
- DOI: 10.1016/j.febslet.2011.03.021
Circadian disruption and SCN control of energy metabolism
Abstract
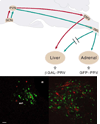
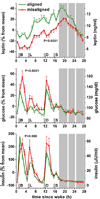
In this review we first present the anatomical pathways used by the suprachiasmatic nuclei to enforce its rhythmicity onto the body, especially its energy homeostatic system. The experimental data show that by activating the orexin system at the start of the active phase, the biological clock not only ensures that we wake up on time, but also that our glucose metabolism and cardiovascular system are prepared for increased activity. The drawback of such a highly integrated system, however, becomes visible when our daily lives are not fully synchronized with the environment. Thus, in addition to increased physical activity and decreased intake of high-energy food, also a well-lighted and fully resonating biological clock may help to withstand the increasing "diabetogenic" pressure of today's 24/7 society.
Copyright © 2011 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures




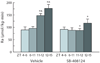
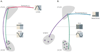
References
-
- Moore RM. Entrainment pathways and the functional organization of the circadian system. Prog. Brain Res. 1996;111:103–119. - PubMed
-
- Challet E, Pévet P. Interactions between photic and nonphotic stimuli to synchronize the master circadian clock in mammals. Front. Biosci. 2003;8:S246–S257. - PubMed
-
- Jin XW, Shearman LP, Weaver DR, Zylka MJ, De Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. - PubMed
-
- Hahm SH, Eiden LE. Five discrete cis-active domains direct cell type-specific transcription of the vasoactive intestinal peptide (VIP) gene. J. Biol. Chem. 1998;273:17086–17094. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

