Glycine receptor channels in spinal motoneurons are abnormal in a transgenic mouse model of amyotrophic lateral sclerosis
- PMID: 21414903
- PMCID: PMC3081715
- DOI: 10.1523/JNEUROSCI.2475-10.2011
Glycine receptor channels in spinal motoneurons are abnormal in a transgenic mouse model of amyotrophic lateral sclerosis
Abstract
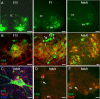
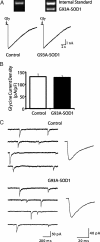
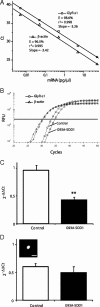
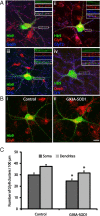
Amyotrophic lateral sclerosis (ALS) is a rapidly evolving and fatal adult-onset neurological disease characterized by progressive degeneration of motoneurons. Our previous study showed that glycinergic innervation of spinal motoneurons is deficient in an ALS mouse model expressing a mutant form of human superoxide dismutase-1 with a Gly93→Ala substitution (G93A-SOD1). In this study, we have examined, using whole-cell patch-clamp recordings, glycine receptor (GlyR)-mediated currents in spinal motoneurons from these transgenic mice. We developed a dissociated spinal cord culture model using embryonic transgenic mice expressing enhanced green fluorescent protein (eGFP) driven by the Hb9 promoter. Motoneurons were identified as Hb9-eGFP-expressing (Hb9-eGFP(+)) neurons with a characteristic morphology. To examine GlyRs in ALS motoneurons, we bred G93A-SOD1 mice to Hb9-eGFP mice and compared glycine-evoked currents in cultured Hb9-eGFP(+) motoneurons prepared from G93A-SOD1 embryos and from their nontransgenic littermates. Glycine-evoked current density was significantly smaller in the G93A-SOD1 motoneurons compared with control. Furthermore, the averaged current densities of spontaneous glycinergic miniature IPSCs (mIPSCs) were significantly smaller in the G93A-SOD1 motoneurons than in control motoneurons. No significant differences in GABA-induced currents and GABAergic mIPSCs were observed between G93A-SOD1 and control motoneurons. Quantitative single-cell reverse transcription-PCR found lower GlyRα1 subunit mRNA expression in G93A-SOD1 motoneurons, indicating that the reduction of GlyR current may result from the downregulation of GlyR mRNA expression in motoneurons. Immunocytochemistry demonstrated a decrease of surface postsynaptic GlyR on G93A-SOD1 motoneurons. Our study suggests that selective alterations in GlyR function contribute to inhibitory insufficiency in motoneurons early in the disease process of ALS.
Figures



References
-
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. - PubMed
-
- Avossa D, Grandolfo M, Mazzarol F, Zatta M, Ballerini L. Early signs of motoneuron vulnerability in a disease model system: characterization of transverse slice cultures of spinal cord isolated from embryonic ALS mice. Neuroscience. 2006;138:1179–1194. - PubMed
-
- Betz H, Kuhse J, Fischer M, Schmieden V, Laube B, Kuryatov A, Langosch D, Meyer G, Bormann J, Rundström N, Matzenbach B, Kirsch J, Ramming M. Structure, diversity and synaptic localization of inhibitory glycine receptors. J Physiol Paris. 1994;88:243–248. - PubMed
-
- Bories C, Amendola J, Lamotte d'Incamps B, Durand J. Early electrophysiological abnormalities in lumbar motoneurons in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2007;25:451–459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
