Active expiration induced by excitation of ventral medulla in adult anesthetized rats
- PMID: 21414911
- PMCID: PMC3142740
- DOI: 10.1523/JNEUROSCI.5338-10.2011
Active expiration induced by excitation of ventral medulla in adult anesthetized rats
Abstract
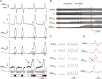
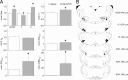
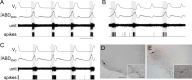
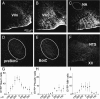
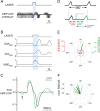
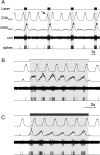
Data from perinatal and juvenile rodents support our hypothesis that the preBötzinger complex generates inspiratory rhythm and the retrotrapezoid nucleus-parafacial respiratory group (RTN/pFRG) generates active expiration (AE). Although the role of the RTN/pFRG in adulthood is disputed, we hypothesized that its rhythmogenicity persists but is typically silenced by synaptic inhibition. We show in adult anesthetized rats that local pharmacological disinhibition or optogenetic excitation of the RTN/pFRG can generate AE and transforms previously silent RTN/pFRG neurons into rhythmically active cells whose firing is correlated with late-phase active expiration. Brief excitatory stimuli also reset the respiratory rhythm, indicating strong coupling of AE to inspiration. The AE network location in adult rats overlaps with the perinatal pFRG and appears lateral to the chemosensitive region of adult RTN. We suggest that (1) the RTN/pFRG contains a conditional oscillator that generates AE, and (2) at rest and in anesthesia, synaptic inhibition of RTN/pFRG suppresses AE.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
