Altered neural responses to sounds in primate primary auditory cortex during slow-wave sleep
- PMID: 21414918
- PMCID: PMC3758555
- DOI: 10.1523/JNEUROSCI.4920-10.2011
Altered neural responses to sounds in primate primary auditory cortex during slow-wave sleep
Abstract
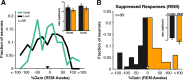
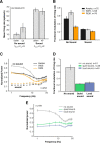
How sounds are processed by the brain during sleep is an important question for understanding how we perceive the sensory environment in this unique behavioral state. While human behavioral data have indicated selective impairments of sound processing during sleep, brain imaging and neurophysiology studies have reported that overall neural activity in auditory cortex during sleep is surprisingly similar to that during wakefulness. This responsiveness to external stimuli leaves open the question of how neural responses during sleep differ, if at all, from wakefulness. Using extracellular neural recordings in the primary auditory cortex of naturally sleeping common marmosets, we show that slow-wave sleep (SWS) alters neural responses in the primate auditory cortex in two specific ways. SWS reduced the sensitivity of auditory cortex such that quiet sounds elicited weak responses in SWS compared with wakefulness, while loud sounds evoked similar responses in SWS and wakefulness. Furthermore, SWS reduced the extent of sound-evoked response suppression. This pattern of alterations was not observed during rapid eye movement sleep and could not be easily explained by the presence of slow rhythms in SWS. The alteration of excitatory and inhibitory responses during SWS suggests limitations in auditory processing and provides novel insights for understanding why certain sounds are processed while others are missed during deep sleep.
Figures

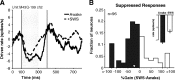
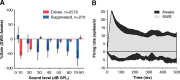
References
-
- Bastuji H, Perrin F, Garcia-Larrea L. Semantic analysis of auditory input during sleep: studies with event related potentials. Int J Psychophysiol. 2002;46:243–255. - PubMed
-
- Bonnet MH. Performance during sleep. In: Webb WB, editor. Biological rhythms, sleep and performance. Chichester, UK: Wiley; 1982. pp. 205–237.
-
- Brugge JF, Merzenich MM. Responses of neurons in auditory cortex of the macaque monkey to monaural and binaural stimulation. J Neurophysiol. 1973;36:1138–1158. - PubMed
-
- Carskadon MA, Rechtschaffen A. Monitoring and staging human sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine. Ed 3. Philadelphia: W.B. Saunders; 2000. pp. 1197–1215.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
