Linking topography to tonotopy in the mouse auditory thalamocortical circuit
- PMID: 21414920
- PMCID: PMC3073837
- DOI: 10.1523/JNEUROSCI.5333-10.2011
Linking topography to tonotopy in the mouse auditory thalamocortical circuit
Abstract
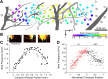
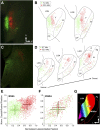
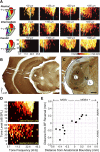
The mouse sensory neocortex is reported to lack several hallmark features of topographic organization such as ocular dominance and orientation columns in primary visual cortex or fine-scale tonotopy in primary auditory cortex (AI). Here, we re-examined the question of auditory functional topography by aligning ultra-dense receptive field maps from the auditory cortex and thalamus of the mouse in vivo with the neural circuitry contained in the auditory thalamocortical slice in vitro. We observed precisely organized tonotopic maps of best frequency (BF) in the middle layers of AI and the anterior auditory field as well as in the ventral and medial divisions of the medial geniculate body (MGBv and MGBm, respectively). Tracer injections into distinct zones of the BF map in AI retrogradely labeled topographically organized MGBv projections and weaker, mixed projections from MGBm. Stimulating MGBv along the tonotopic axis in the slice produced an orderly shift of voltage-sensitive dye (VSD) signals along the AI tonotopic axis, demonstrating topography in the mouse thalamocortical circuit that is preserved in the slice. However, compared with BF maps of neuronal spiking activity, the topographic order of subthreshold VSD maps was reduced in layer IV and even further degraded in layer II/III. Therefore, the precision of AI topography varies according to the source and layer of the mapping signal. Our findings further bridge the gap between in vivo and in vitro approaches for the detailed cellular study of auditory thalamocortical circuit organization and plasticity in the genetically tractable mouse model.
Figures





Similar articles
-
Evidence for nonreciprocal organization of the mouse auditory thalamocortical-corticothalamic projection systems.J Comp Neurol. 2008 Mar 10;507(2):1209-27. doi: 10.1002/cne.21602. J Comp Neurol. 2008. PMID: 18181153
-
Independent tonotopy and thalamocortical projection patterns in two adjacent parts of the classical primary auditory cortex in mice.Neurosci Lett. 2017 Jan 10;637:26-30. doi: 10.1016/j.neulet.2016.11.062. Epub 2016 Nov 30. Neurosci Lett. 2017. PMID: 27914952
-
Parallel thalamocortical pathways for echolocation and passive sound localization in a gleaning bat, Antrozous pallidus.J Comp Neurol. 2007 Jan 10;500(2):322-38. doi: 10.1002/cne.21178. J Comp Neurol. 2007. PMID: 17111376
-
Reconsidering Tonotopic Maps in the Auditory Cortex and Lemniscal Auditory Thalamus in Mice.Front Neural Circuits. 2017 Feb 28;11:14. doi: 10.3389/fncir.2017.00014. eCollection 2017. Front Neural Circuits. 2017. PMID: 28293178 Free PMC article. Review.
-
Functional organization of lemniscal and nonlemniscal auditory thalamus.Exp Brain Res. 2003 Dec;153(4):543-9. doi: 10.1007/s00221-003-1611-5. Epub 2003 Aug 23. Exp Brain Res. 2003. PMID: 12937877 Review.
Cited by
-
Primary auditory thalamus relays directly to cortical layer 1 interneurons.bioRxiv [Preprint]. 2024 Jul 18:2024.07.16.603741. doi: 10.1101/2024.07.16.603741. bioRxiv. 2024. Update in: iScience. 2025 Jun 24;28(8):112652. doi: 10.1016/j.isci.2025.112652. PMID: 39071266 Free PMC article. Updated. Preprint.
-
Plasticity of Multidimensional Receptive Fields in Core Rat Auditory Cortex Directed by Sound Statistics.Neuroscience. 2021 Jul 15;467:150-170. doi: 10.1016/j.neuroscience.2021.04.028. Epub 2021 May 2. Neuroscience. 2021. PMID: 33951506 Free PMC article.
-
Transient Subgranular Hyperconnectivity to L2/3 and Enhanced Pairwise Correlations During the Critical Period in the Mouse Auditory Cortex.Cereb Cortex. 2020 Mar 14;30(3):1914-1930. doi: 10.1093/cercor/bhz213. Cereb Cortex. 2020. PMID: 31667495 Free PMC article.
-
A Missing Connection: A Review of the Macrostructural Anatomy and Tractography of the Acoustic Radiation.Front Neuroanat. 2019 Mar 7;13:27. doi: 10.3389/fnana.2019.00027. eCollection 2019. Front Neuroanat. 2019. PMID: 30899216 Free PMC article. Review.
-
Durability of high surface area platinum deposits on microelectrode arrays for acute neural recordings.J Mater Sci Mater Med. 2014 Mar;25(3):931-40. doi: 10.1007/s10856-013-5114-z. Epub 2013 Dec 7. J Mater Sci Mater Med. 2014. PMID: 24318022
References
-
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–286. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
