Behavioral choice between conflicting alternatives is regulated by a receptor guanylyl cyclase, GCY-28, and a receptor tyrosine kinase, SCD-2, in AIA interneurons of Caenorhabditis elegans
- PMID: 21414922
- PMCID: PMC6623760
- DOI: 10.1523/JNEUROSCI.4691-10.2011
Behavioral choice between conflicting alternatives is regulated by a receptor guanylyl cyclase, GCY-28, and a receptor tyrosine kinase, SCD-2, in AIA interneurons of Caenorhabditis elegans
Abstract
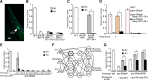
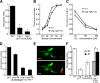
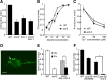
Animals facing conflicting sensory cues make a behavioral choice between competing alternatives through integration of the sensory cues. Here, we performed a genetic screen to identify genes important for the sensory integration of two conflicting cues, the attractive odorant diacetyl and the aversive stimulus Cu(2+), and found that the membrane-bound guanylyl cyclase GCY-28 and the receptor tyrosine kinase SCD-2 regulate the behavioral choice between these alternatives in Caenorhabditis elegans. The gcy-28 mutants and scd-2 mutants show an abnormal bias in the behavioral choice between the cues, although their responses to each individual cue are similar to those in wild-type animals. Mutants in a gene encoding a cyclic nucleotide gated ion channel, cng-1, also exhibit the defect in sensory integration. Molecular genetic analyses suggested that GCY-28 and SCD-2 regulate sensory integration in AIA interneurons, where the conflicting sensory cues may converge. Genetic ablation or hyperpolarization of AIA interneurons showed nearly the same phenotype as gcy-28 or scd-2 mutants in the sensory integration, although this did not affect the sensory response to each individual cue. In gcy-28 or scd-2 mutants, activation of AIA interneurons is sufficient to restore normal sensory integration. These results suggest that the activity of AIA interneurons regulates the behavioral choice between the alternatives. We propose that GCY-28 and SCD-2 regulate sensory integration by modulating the activity of AIA interneurons.
Figures


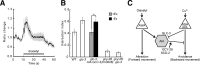
Similar articles
-
Mutations in the guanylate cyclase gcy-28 neuronally dissociate naïve attraction and memory retrieval.Eur J Neurosci. 2018 Dec;48(11):3367-3378. doi: 10.1111/ejn.14221. Epub 2018 Nov 19. Eur J Neurosci. 2018. PMID: 30362188
-
A novel zf-MYND protein, CHB-3, mediates guanylyl cyclase localization to sensory cilia and controls body size of Caenorhabditis elegans.PLoS Genet. 2010 Nov 24;6(11):e1001211. doi: 10.1371/journal.pgen.1001211. PLoS Genet. 2010. PMID: 21124861 Free PMC article.
-
Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans.Genetics. 2006 Apr;172(4):2239-52. doi: 10.1534/genetics.105.050013. Epub 2006 Jan 16. Genetics. 2006. PMID: 16415369 Free PMC article.
-
Behavioral genetics: guanylyl cyclase prompts worms to party.Curr Biol. 2004 Aug 24;14(16):R657-8. doi: 10.1016/j.cub.2004.08.011. Curr Biol. 2004. PMID: 15324682 Review.
-
[Mechanisms of molecular and neural network for thermotaxis in C. elegans].Tanpakushitsu Kakusan Koso. 2007 Mar;52(3):205-13. Tanpakushitsu Kakusan Koso. 2007. PMID: 17352184 Review. Japanese. No abstract available.
Cited by
-
Worms love Coffee too! Characterizing the neural substrates that regulate odor-guided responses to coffee.MicroPubl Biol. 2024 Oct 18;2024:10.17912/micropub.biology.001242. doi: 10.17912/micropub.biology.001242. eCollection 2024. MicroPubl Biol. 2024. PMID: 39502422 Free PMC article.
-
Signal Decoding for Glutamate Modulating Egg Laying Oppositely in Caenorhabditis elegans under Varied Environmental Conditions.iScience. 2020 Sep 19;23(10):101588. doi: 10.1016/j.isci.2020.101588. eCollection 2020 Oct 23. iScience. 2020. PMID: 33089099 Free PMC article.
-
Mutations in the guanylate cyclase gcy-28 neuronally dissociate naïve attraction and memory retrieval.Eur J Neurosci. 2018 Dec;48(11):3367-3378. doi: 10.1111/ejn.14221. Epub 2018 Nov 19. Eur J Neurosci. 2018. PMID: 30362188
-
Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology.Nat Rev Cancer. 2013 Oct;13(10):685-700. doi: 10.1038/nrc3580. Nat Rev Cancer. 2013. PMID: 24060861 Review.
-
Cross-modulation of homeostatic responses to temperature, oxygen and carbon dioxide in C. elegans.PLoS Genet. 2013;9(12):e1004011. doi: 10.1371/journal.pgen.1004011. Epub 2013 Dec 19. PLoS Genet. 2013. PMID: 24385919 Free PMC article.
References
-
- Abdelalim EM, Masuda C, Tooyama I. Expression of natriuretic peptide-activated guanylate cyclases by cholinergic and dopaminergic amacrine cells of the rat retina. Peptides. 2008;29:622–628. - PubMed
-
- Amet LE, Lauri SE, Hienola A, Croll SD, Lu Y, Levorse JM, Prabhakaran B, Taira T, Rauvala H, Vogt TF. Enhanced hippocampal long-term potentiation in mice lacking heparin-binding growth-associated molecule. Mol Cell Neurosci. 2001;17:1014–1024. - PubMed
-
- Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. - PubMed
-
- Baude EJ, Arora VK, Yu S, Garbers DL, Wedel BJ. The cloning of a Caenorhabditis elegans guanylyl cyclase and the construction of a ligand-sensitive mammalian/nematode chimeric receptor. J Biol Chem. 1997;272:16035–16039. - PubMed
-
- Bessereau JL, Wright A, Williams DC, Schuske K, Davis MW, Jorgensen EM. Mobilization of a Drosophila transposon in the Caenorhabditis elegans germ line. Nature. 2001;413:70–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
