Axonal branching patterns as sources of delay in the mammalian auditory brainstem: a re-examination
- PMID: 21414923
- PMCID: PMC3157295
- DOI: 10.1523/JNEUROSCI.5175-10.2011
Axonal branching patterns as sources of delay in the mammalian auditory brainstem: a re-examination
Abstract
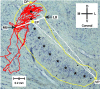
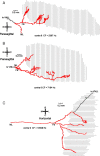
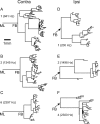
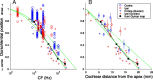
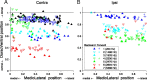
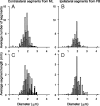
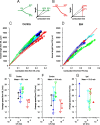
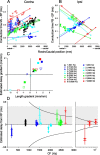
In models of temporal processing, time delays incurred by axonal propagation of action potentials play a prominent role. A pre-eminent model of temporal processing in audition is the binaural model of Jeffress (1948), which has dominated theories regarding our acute sensitivity to interaural time differences (ITDs). In Jeffress' model, a binaural cell is maximally active when the ITD is compensated by an internal delay, which brings the inputs from left and right ears in coincidence, and which would arise from axonal branching patterns of monaural input fibers. By arranging these patterns in systematic and opposite ways for the ipsilateral and contralateral inputs, a range of length differences, and thereby of internal delays, is created so that the ITD is transformed into a spatial activation pattern along the binaural nucleus. We reanalyze single, labeled, and physiologically characterized axons of spherical bushy cells of the cat anteroventral cochlear nucleus, which project to binaural coincidence detectors in the medial superior olive (MSO). The reconstructions largely confirm the observations of two previous reports, but several features are observed that are inconsistent with Jeffress' model. We found that ipsilateral projections can also form a caudally directed delay line pattern, which would counteract delays incurred by caudally directed contralateral projections. Comparisons of estimated axonal delays with binaural physiological data indicate that the suggestive anatomical patterns cannot account for the frequency-dependent distribution of best delays in the cat. Surprisingly, the tonotopic distribution of the afferent endings indicate that low characteristic frequencies are under-represented rather than over-represented in the MSO.
Figures






Similar articles
-
Oscillatory dipoles as a source of phase shifts in field potentials in the mammalian auditory brainstem.J Neurosci. 2010 Oct 6;30(40):13472-87. doi: 10.1523/JNEUROSCI.0294-10.2010. J Neurosci. 2010. PMID: 20926673 Free PMC article.
-
Projections of physiologically characterized spherical bushy cell axons from the cochlear nucleus of the cat: evidence for delay lines to the medial superior olive.J Comp Neurol. 1993 May 8;331(2):245-60. doi: 10.1002/cne.903310208. J Comp Neurol. 1993. PMID: 8509501
-
Envelope coding in the lateral superior olive. II. Characteristic delays and comparison with responses in the medial superior olive.J Neurophysiol. 1996 Oct;76(4):2137-56. doi: 10.1152/jn.1996.76.4.2137. J Neurophysiol. 1996. PMID: 8899590
-
Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei.Brain Res Bull. 2003 Jun 15;60(5-6):457-74. doi: 10.1016/s0361-9230(03)00050-9. Brain Res Bull. 2003. PMID: 12787867 Review.
-
The ion channels and synapses responsible for the physiological diversity of mammalian lower brainstem auditory neurons.Hear Res. 2019 May;376:33-46. doi: 10.1016/j.heares.2018.12.011. Epub 2018 Dec 26. Hear Res. 2019. PMID: 30606624 Review.
Cited by
-
In vivo coincidence detection in mammalian sound localization generates phase delays.Nat Neurosci. 2015 Mar;18(3):444-52. doi: 10.1038/nn.3948. Epub 2015 Feb 9. Nat Neurosci. 2015. PMID: 25664914 Free PMC article.
-
A model of the medial superior olive explains spatiotemporal features of local field potentials.J Neurosci. 2014 Aug 27;34(35):11705-22. doi: 10.1523/JNEUROSCI.0175-14.2014. J Neurosci. 2014. PMID: 25164666 Free PMC article.
-
The Physiological Basis and Clinical Use of the Binaural Interaction Component of the Auditory Brainstem Response.Ear Hear. 2016 Sep-Oct;37(5):e276-e290. doi: 10.1097/AUD.0000000000000301. Ear Hear. 2016. PMID: 27232077 Free PMC article. Review.
-
Neural Maps of Interaural Time Difference in the American Alligator: A Stable Feature in Modern Archosaurs.J Neurosci. 2019 May 15;39(20):3882-3896. doi: 10.1523/JNEUROSCI.2989-18.2019. Epub 2019 Mar 18. J Neurosci. 2019. PMID: 30886018 Free PMC article.
-
Auditory Brainstem Responses in Two Closely Related Peromyscus Species (Peromyscus leucopus and Peromyscus maniculatus).bioRxiv [Preprint]. 2025 Apr 9:2024.12.09.627419. doi: 10.1101/2024.12.09.627419. bioRxiv. 2025. Update in: J Acoust Soc Am. 2025 Jun 1;157(6):4559-4572. doi: 10.1121/10.0036945. PMID: 39713444 Free PMC article. Updated. Preprint.
References
-
- Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981;29:775. - PubMed
-
- Bonham BH, Lewis ER. Localization by interaural time difference (ITD): effects of interaural frequency mismatch. J Acoust Soc Am. 1999;106:281–290. - PubMed
-
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–547. - PubMed
-
- Brownell WE. Organization of the cat trapezoid body and the discharge characteristics of its fibers. Brain Res. 1975;94:413–433. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
