Certolizumab pegol plus methotrexate provides broad relief from the burden of rheumatoid arthritis: analysis of patient-reported outcomes from the RAPID 2 trial
- PMID: 21415050
- PMCID: PMC3086050
- DOI: 10.1136/ard.2010.143586
Certolizumab pegol plus methotrexate provides broad relief from the burden of rheumatoid arthritis: analysis of patient-reported outcomes from the RAPID 2 trial
Abstract
Objective: To assess the impact of certolizumab pegol (CZP) on patient-reported outcomes (PROs) in rheumatoid arthritis (RA), and to interpret these results using number needed to treat (NNT), and associations between PRO responses and longer term outcomes.
Methods: A total of 619 patients with active RA were randomised to CZP 200 or 400 mg, or placebo plus methotrexate (MTX). PROs assessed included pain, patient's global assessment of disease activity (PtGA), physical function, fatigue and health-related quality of life. Treatment impact on PROs, NNT to achieve simultaneous improvements in multiple PROs and correlations between PROs were calculated. Times to onset of improvements greater than or equal to minimum clinically important differences (MCIDs) in pain as a determinant of clinical outcomes at week 24 were compared between week 6 and 12 responders, and in patients with improvements in pain ≥ MCID at week 12 (week 12 responders/non-responders).
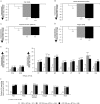
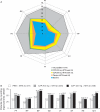
Results: CZP 200 and 400 mg plus MTX were associated with rapid, clinically meaningful improvements in all PROs. The NNT for subjects to report changes ≥MCID in up to five PROs was two to three, and five for all six PROs (pain, PtGA, physical function, fatigue and short-form 36-item Physical and Mental Component Summary Scores). More patients with improvements ≥MCID in pain at week 6 than those at week 12 had lower disease activity at week 24. Week 12 pain responders had better clinical outcomes at week 24 than non-responders.
Conclusions: The data demonstrate that CZP provides broad relief from the burden of RA. Trial registration number NCT00160602.
Conflict of interest statement
Figures
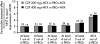
References
-
- Strand V, Singh JA. Newer biological agents in rheumatoid arthritis: impact on health-related quality of life and productivity. Drugs 2010;70:121–45 - PubMed
-
- Keystone E, Heijde D, Mason D, Jr, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 2008;58:3319–29 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

