Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies
- PMID: 21415101
- PMCID: PMC3230110
- DOI: 10.1136/bmj.d1309
Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies
Abstract
Objective: To determine the comparative effects of the thiazolidinediones (rosiglitazone and pioglitazone) on myocardial infarction, congestive heart failure, and mortality in patients with type 2 diabetes.
Design: Systematic review and meta-analysis of observational studies.
Data sources: Searches of Medline and Embase in September 2010.
Study selection: Observational studies that directly compared the risk of cardiovascular outcomes for rosiglitazone and pioglitazone among patients with type 2 diabetes mellitus were included.
Data extraction: Random effects meta-analysis (inverse variance method) was used to calculate the odds ratios for cardiovascular outcomes with thiazolidinedione use. The I(2 )statistic was used to assess statistical heterogeneity.
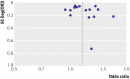
Results: Cardiovascular outcomes from 16 observational studies (4 case-control studies and 12 retrospective cohort studies), including 810,000 thiazolidinedione users, were evaluated after a detailed review of 189 citations. Compared with pioglitazone, use of rosiglitazone was associated with a statistically significant increase in the odds of myocardial infarction (n = 15 studies; odds ratio 1.16, 95% confidence interval 1.07 to 1.24; P < 0.001; I(2) = 46%), congestive heart failure (n = 8; 1.22, 1.14 to 1.31; P < 0.001; I(2) = 37%), and death (n = 8; 1.14, 1.09 to 1.20; P < 0.001; I(2) = 0%). Numbers needed to treat to harm (NNH), depending on the population at risk, suggest 170 excess myocardial infarctions, 649 excess cases of heart failure, and 431 excess deaths for every 100,000 patients who receive rosiglitazone rather than pioglitazone.
Conclusion: Among patients with type 2 diabetes, use of rosiglitazone is associated with significantly higher odds of congestive heart failure, myocardial infarction, and death relative to pioglitazone in real world settings.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures



Comment in
-
What have we learnt from the rosiglitazone saga?BMJ. 2011 Mar 17;342:d1354. doi: 10.1136/bmj.d1354. BMJ. 2011. PMID: 21415102 No abstract available.
-
Rosiglitazone saga. Demonisation of rosiglitazone.BMJ. 2011 Apr 21;342:d2571; author reply d2573. doi: 10.1136/bmj.d2571. BMJ. 2011. PMID: 21511791 No abstract available.
-
ACP Journal Club. Review: rosiglitazone increases risk for cardiovascular outcomes and mortality compared with pioglitazone in type 2 diabetes.Ann Intern Med. 2011 Aug 16;155(4):JC2-12. doi: 10.7326/0003-4819-155-4-201108160-02012. Ann Intern Med. 2011. PMID: 21844545 No abstract available.
References
-
- Gale EA. Lessons from the glitazones: a story of drug development. Lancet 2001;357:1870-5. - PubMed
-
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005;294:2581-6. - PubMed
-
- Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med 2010;170:1191-201. - PubMed
-
- Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA 2007;298:1189-95. - PubMed
-
- Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 2007;298:1180-8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
