Remote control of neuronal signaling
- PMID: 21415127
- PMCID: PMC3082452
- DOI: 10.1124/pr.110.003020
Remote control of neuronal signaling
Abstract
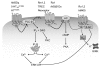
A significant challenge for neuroscientists is to determine how both electrical and chemical signals affect the activity of cells and circuits and how the nervous system subsequently translates that activity into behavior. Remote, bidirectional manipulation of those signals with high spatiotemporal precision is an ideal approach to addressing that challenge. Neuroscientists have recently developed a diverse set of tools that permit such experimental manipulation with varying degrees of spatial, temporal, and directional control. These tools use light, peptides, and small molecules to primarily activate ion channels and G protein-coupled receptors (GPCRs) that in turn activate or inhibit neuronal firing. By monitoring the electrophysiological, biochemical, and behavioral effects of such activation/inhibition, researchers can better understand the links between brain activity and behavior. Here, we review the tools that are available for this type of experimentation. We describe the development of the tools and highlight exciting in vivo data. We focus primarily on designer GPCRs (receptors activated solely by synthetic ligands, designer receptors exclusively activated by designer drugs) and microbial opsins (e.g., channelrhodopsin-2, halorhodopsin, Volvox carteri channelrhodopsin) but also describe other novel techniques that use orthogonal receptors, caged ligands, allosteric modulators, and other approaches. These tools differ in the direction of their effect (activation/inhibition, hyperpolarization/depolarization), their onset and offset kinetics (milliseconds/minutes/hours), the degree of spatial resolution they afford, and their invasiveness. Although none of these tools is perfect, each has advantages and disadvantages, which we describe, and they are all still works in progress. We conclude with suggestions for improving upon the existing tools.
Figures

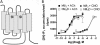

References
-
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. (2009) Temporally precise in vivo control of intracellular signalling. Nature 458:1025–1029 - PubMed
-
- Alvarez-Curto E, Ward RJ, Pediani JD, Milligan G. (2010) Ligand regulation of the quaternary organization of cell surface M3 muscarinic acetylcholine receptors analyzed by fluorescence resonance energy transfer (FRET) imaging and homogeneous time-resolved FRET. J Biol Chem 285:23318–23330 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

