Autologous transplantation gives encouraging results for young adults with favorable-risk acute myeloid leukemia, but is not improved with gemtuzumab ozogamicin
- PMID: 21415269
- PMCID: PMC3109705
- DOI: 10.1182/blood-2010-09-309229
Autologous transplantation gives encouraging results for young adults with favorable-risk acute myeloid leukemia, but is not improved with gemtuzumab ozogamicin
Abstract
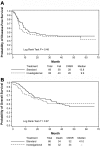
We report the results of a prospective, randomized phase 3 trial evaluating the use of gemtuzumab ozogamicin (GO) in an intensive consolidation approach in 657 patients 17-60 years of age. Patients in first complete remission (CR1) after cytarabine and standard- or high-dose daunorubicin induction received 2 cycles of consolidation with high-dose cytarabine followed by peripheral blood progenitor cell collection. The 352 patients who entered consolidation were randomized to receive GO (n = 132) or not (n = 138) and then proceeded to autologous hematopoietic cell transplantation (HCT). GO was given to 67 patients. Median follow-up was 50.9 months. Results of the intention-to-treat analysis demonstrated a 4-year disease-free survival (DFS) of 33.6% versus 35.9% (P = .54) and an overall survival (OS) of 41.3% versus 41.9% (P = .52) for those randomized to receive GO versus no GO, respectively. Patients with favorable- and intermediate-risk acute myeloid leukemia (AML) treated with high-dose daunorubicin and autologous HCT had 4-year DFS rates of 60% and 40% and OS rates of 80% and 49.3%, respectively. For younger AML patients in CR1, autologous HCT should be considered in favorable- and intermediate-cytogenetic risk patients who do not have an allogeneic donor. The addition of a single dose of GO in this setting did not improve outcomes.
Trial registration: ClinicalTrials.gov NCT00049517.
Figures





References
-
- Burnett AK, Goldstone AH, Stevens RM, et al. Randomised comparison of addition of autologous bone-marrow transplantation to intensive chemotherapy for acute myeloid leukaemia in first remission: results of MRC AML 10 trial. UK Medical Research Council Adult and Children's Leukaemia Working Parties. Lancet. 1998;351(9104):700–708. - PubMed
-
- Zittoun RA, Mandelli F, Willemze R, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med. 1995;332(4):217–223. - PubMed
-
- Cassileth PA, Lee SJ, Litzow MR, et al. Intensified induction chemotherapy in adult acute myeloid leukemia followed by high-dose chemotherapy and autologous peripheral blood stem cell transplantation: an Eastern Cooperative Oncology Group trial (E4995). Leuk Lymphoma. 2005;46(1):55–61. - PubMed
-
- Keating A, Kukreja M, Silva GTd, et al. Similar 5-year survival after peripheral blood autotransplants (AutoPB) versus hLA matched sibling myeloablative transplants (AlloBMT) for acute myeloid leukemia (AML) in first complete remission (CR1) [Abstract]. Blood. 2008;112(11):2168. - PubMed
-
- Linker CA, Ries CA, Damon LE, et al. Autologous stem cell transplantation for acute myeloid leukemia in first remission. Biol Blood Marrow Transplant. 2000;6(1):50–57. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- CA14548/CA/NCI NIH HHS/United States
- U10 CA013650/CA/NCI NIH HHS/United States
- CA66636/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- CA13650/CA/NCI NIH HHS/United States
- U10 CA017145/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- CA17145/CA/NCI NIH HHS/United States
- CA15488/CA/NCI NIH HHS/United States
- U10 CA015488/CA/NCI NIH HHS/United States
- U10 CA014548/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- U24 CA114737/CA/NCI NIH HHS/United States
- CA23318/CA/NCI NIH HHS/United States

