Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT
- PMID: 21415383
- PMCID: PMC3292330
- DOI: 10.2337/db10-1392
Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT
Abstract
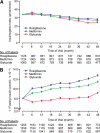
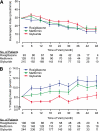
Objective: ADOPT (A Diabetes Outcome Progression Trial) demonstrated that initial monotherapy with rosiglitazone provided superior durability of glycemic control compared with metformin and glyburide in patients with recently diagnosed type 2 diabetes. Herein, we examine measures of β-cell function and insulin sensitivity from an oral glucose tolerance test (OGTT) over a 4-year period among the three treatments.
Research design and methods: Recently diagnosed, drug-naïve patients with type 2 diabetes (4,360 total) were treated for a median of 4.0 years with rosiglitazone, metformin, or glyburide and were examined with periodic metabolic testing using an OGTT.
Results: Measures of β-cell function and insulin sensitivity from an OGTT showed more favorable changes over time with rosiglitazone versus metformin or glyburide. Persistent improvements were seen in those who completed 4 years of monotherapy and marked deterioration of β-cell function in those who failed to maintain adequate glucose control with initial monotherapy.
Conclusions: The favorable combined changes in β-cell function and insulin sensitivity over time with rosiglitazone appear to be responsible for its superior glycemic durability over metformin and glyburide as initial monotherapy in type 2 diabetes.
Figures


References
-
- UK Prospective Diabetes Study Group U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995;44:1249–1258 - PubMed
-
- Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA 2002;287:360–372 - PubMed
-
- Viberti G, Kahn SE, Greene DA, et al. A diabetes outcome progression trial (ADOPT): an international multicenter study of the comparative efficacy of rosiglitazone, glyburide, and metformin in recently diagnosed type 2 diabetes. Diabetes Care 2002;25:1737–1743 - PubMed
-
- Kahn SE, Haffner SM, Heise MA, et al. ; ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 - PubMed
-
- Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med 1994;11:286–292 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

