The ephrin A1-EphA2 system promotes cardiac stem cell migration after infarction
- PMID: 21415392
- PMCID: PMC3102645
- DOI: 10.1161/CIRCRESAHA.110.239459
The ephrin A1-EphA2 system promotes cardiac stem cell migration after infarction
Erratum in
-
Correction.Circ Res. 2015 Dec 4;117(12):e129. doi: 10.1161/RES.0000000000000084. Circ Res. 2015. PMID: 26635383 No abstract available.
-
Correction.Circ Res. 2017 Jan 20;120(2):e1. doi: 10.1161/RES.0000000000000131. Circ Res. 2017. PMID: 28104775 No abstract available.
Retraction in
-
Retraction of: The Ephrin A1-EphA2 System Promotes Cardiac Stem Cell Migration After Infarction.Circ Res. 2019 Feb 15;124(4):e25. doi: 10.1161/RES.0000000000000249. Circ Res. 2019. PMID: 30582461 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern.Circ Res. 2019 Jan 18;124(2):e4-e5. doi: 10.1161/RES.0000000000000241. Circ Res. 2019. PMID: 30582460 No abstract available.
-
Expression of Concern.Circulation. 2019 Jan 15;139(3):e5-e6. doi: 10.1161/CIR.0000000000000639. Circulation. 2019. PMID: 30615475 No abstract available.
Abstract
Rationale: Understanding the mechanisms that regulate trafficking of human cardiac stem cells (hCSCs) may lead to development of new therapeutic approaches for the failing heart.
Objective: We tested whether the motility of hCSCs in immunosuppressed infarcted animals is controlled by the guidance system that involves the interaction of Eph receptors with ephrin ligands.
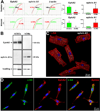
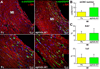
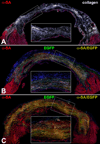
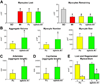
Methods and results: Within the cardiac niches, cardiomyocytes expressed preferentially the ephrin A1 ligand, whereas hCSCs possessed the EphA2 receptor. Treatment of hCSCs with ephrin A1 resulted in the rapid internalization of the ephrin A1-EphA2 complex, posttranslational modifications of Src kinases, and morphological changes consistent with the acquisition of a motile cell phenotype. Ephrin A1 enhanced the motility of hCSCs in vitro, and their migration in vivo following acute myocardial infarction. At 2 weeks after infarction, the volume of the regenerated myocardium was 2-fold larger in animals injected with ephrin A1-activated hCSCs than in animals receiving control hCSCs; this difference was dictated by a greater number of newly formed cardiomyocytes and coronary vessels. The increased recovery in myocardial mass with ephrin A1-treated hCSCs was characterized by further restoration of cardiac function and by a reduction in arrhythmic events.
Conclusions: Ephrin A1 promotes the motility of EphA2-positive hCSCs, facilitates their migration to the area of damage, and enhances cardiac repair. Thus, in situ stimulation of resident hCSCs with ephrin A1 or their ex vivo activation before myocardial delivery improves cell targeting to sites of injury, possibly providing a novel strategy for the management of the diseased heart.
Figures








References
-
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. - PubMed
-
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. - PMC - PubMed
-
- Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG017042/AG/NIA NIH HHS/United States
- R01 HL111183/HL/NHLBI NIH HHS/United States
- R01 HL075480/HL/NHLBI NIH HHS/United States
- P01 HL092868/HL/NHLBI NIH HHS/United States
- R01 AG037490/AG/NIA NIH HHS/United States
- R01 HL091021/HL/NHLBI NIH HHS/United States
- R01 HL039902/HL/NHLBI NIH HHS/United States
- R01 AG037495/AG/NIA NIH HHS/United States
- R01 AG026107/AG/NIA NIH HHS/United States
- R01 HL065577/HL/NHLBI NIH HHS/United States
- R37 HL081737/HL/NHLBI NIH HHS/United States
- P01 AG023071/AG/NIA NIH HHS/United States
- R21 HL094894/HL/NHLBI NIH HHS/United States
- R01 HL065573/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

