A novel role for calpain in the endothelial dysfunction induced by activation of angiotensin II type 1 receptor signaling
- PMID: 21415394
- PMCID: PMC6159903
- DOI: 10.1161/CIRCRESAHA.110.229393
A novel role for calpain in the endothelial dysfunction induced by activation of angiotensin II type 1 receptor signaling
Abstract
Rationale: The cytosolic protease calpain has been recently implicated in the vascular remodeling of angiotensin II (Ang II) type 1 receptor (AT(1)R) signaling. The role of Ang II/AT(1)R/calpain signaling on endothelial function, an important and early determinant of vascular pathology, remains though totally unknown. Accordingly, we investigated the role of calpain in the endothelial dysfunction of Ang II.
Objective: To demonstrate a mechanistic role for calpain in the endothelial dysfunction induced by Ang II/AT(1)R signaling. To establish endothelial-expressed calpains as an important target of AT(1)R signaling.
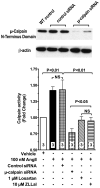
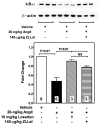
Methods and results: Subchronic administration of nonpressor doses of Ang II to rats and mice significantly increased vascular calpain activity via AT(1)R signaling. Intravital microscopy studies revealed that activation of vascular expressed calpains causes endothelial dysfunction with increased leukocyte-endothelium interactions and albumin permeability in the microcirculation. Western blot and immunohistochemistry studies confirmed that Ang II/AT(1)R signaling preferentially activates the constitutively expressed μ-calpain isoform and demonstrated a calpain-dependent degradation of IκBα, along with upregulation of nuclear factor κB-regulated endothelial cell adhesion molecules. These physiological and biochemical parameters were nearly normalized following inhibition of AT(1)R or calpain in vivo. RNA silencing studies in microvascular endothelial cells, along with knockout and transgenic mouse studies, further confirmed the role of μ-calpain in the endothelial adhesiveness induced by Ang II.
Conclusions: This study uncovers a novel role for calpain in the endothelial dysfunction of Ang II/AT(1)R signaling and establishes the calpain system as a novel molecular target of the vascular protective action of renin-angiotensin system inhibition. Our results may have significant clinical implications in vascular disease.
Figures






References
-
- Fujitani K, Kambayashi J, Sakon M, Ohmi SI, Kawashima S, Yukawa M, Yano Y, Miyoshi H, Ikeda M, Shinoki N, Monden M. Identification of mu-, m-calpains and calpastatin and capture of mu-calpain activation in endothelial cells. Journal of cellular biochemistry. 1997;66:197–209. - PubMed
-
- Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, Baud L. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin ii-induced hypertension. Circulation research. 2008;102:720–728. - PubMed
-
- Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP, 3rd, Howatt DA, Subramanian V, Poduri A, Charnigo R, Cassis LA, Daugherty A. Endothelial cell-specific deficiency of ang ii type 1a receptors attenuates ang ii-induced ascending aortic aneurysms in ldl receptor−/− mice. Circulation research. 2011 - PMC - PubMed
-
- Pastore L, Tessitore A, Martinotti S, Toniato E, Alesse E, Bravi MC, Ferri C, Desideri G, Gulino A, Santucci A. Angiotensin ii stimulates intercellular adhesion molecule-1 (icam-1) expression by human vascular endothelial cells and increases soluble icam-1 release in vivo. Circulation. 1999;100:1646–1652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

