Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier
- PMID: 21415414
- PMCID: PMC3119114
- DOI: 10.1152/ajpgi.00055.2011
Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier
Abstract
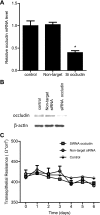
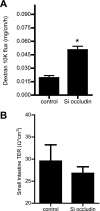
Defective intestinal epithelial tight junction (TJ) barrier has been shown to be an important pathogenic factor contributing to the development of intestinal inflammation. The expression of occludin is markedly decreased in intestinal permeability disorders, including in Crohn's disease, ulcerative colitis, and celiac disease, suggesting that the decrease in occludin expression may play a role in the increase in intestinal permeability. The purpose of this study was to delineate the involvement of occludin in intestinal epithelial TJ barrier by selective knock down of occludin in in vitro (filter-grown Caco-2 monolayers) and in vivo (recycling perfusion of mouse intestine) intestinal epithelial models. Our results indicated that occludin small-interfering RNA (siRNA) transfection causes an increase in transepithelial flux of various-sized probes, including urea, mannitol, inulin, and dextran, across the Caco-2 monolayers, without affecting the transepithelial resistance. The increase in relative flux rate was progressively greater for larger-sized probes, indicating that occludin depletion has the greatest effect on the flux of large macromolecules. siRNA-induced knock down of occludin in mouse intestine in vivo also caused an increase in intestinal permeability to dextran but did not affect intestinal tissue transepithelial resistance. In conclusion, these results show for the first time that occludin depletion in intestinal epithelial cells in vitro and in vivo leads to a selective or preferential increase in macromolecule flux, suggesting that occludin plays a crucial role in the maintenance of TJ barrier through the large-channel TJ pathway, the pathway responsible for the macromolecule flux.
Figures





References
-
- Bamforth SD, Kniesel U, Wolburg H, Engelhardt B, Risau W. A dominant mutant of occludin disrupts tight junction structure and function. J Cell Sci 112: 1879–1888, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

