Cholera toxin - a foe & a friend
- PMID: 21415489
- PMCID: PMC3089046
Cholera toxin - a foe & a friend
Abstract
After De΄s pivotal demonstration in 1959 of a diarrhoeogenic exo-enterotoxin in cell-free culture filtrates from Vibrio cholerae (of classical biotype), much insight has been gained about cholera toxin (CT), which is arguably now the best known of all microbial toxins. The subunit structure and function of CT, its receptor (the GM1 ganglioside), and its effects on the cyclic AMP system and on intestinal secretion were defined in the 1970s, and the essential aspects of the genetic organization in the 1980s. Recent findings have generated additional perspectives. The 3D-crystal structure of CT has been established, the CT-encoding operon has been shown to be carried by a non-lytic bacteriophage, and in depth knowledge has been gained on how the bacterium controls CT gene expression in response to cell density and various environmental signals. The mode of entry into target cells and the intracellular transport of CT are becoming clearer. CT has become the prototype enterotoxin and a widely used tool for elucidating important aspects of cell biology and physiology, e.g., cell membrane receptors, the cyclic AMP system, G proteins, as well as normal and pathological ion transport mechanisms. In immunology, CT has emerged as a potent, widely used experimental adjuvant, and the strong oral-mucosal immunogenicity of the non-toxic B-subunit (CTB) has led to the use of CTB as a protective antigen together with killed vibrios in a widely licensed oral cholera vaccine. CTB has also been shown to promote immunological tolerance against certain types of mucosally co-administered antigens, preferably tissue antigens linked to the CTB molecule; this has stimulated research and development to use CTB in this context for treatment of autoimmune and allergic diseases. In summary, in the 50 years after De΄s discovery of CT, this molecule has emerged from being the cholera patient΄s "foe" to also becoming a highly useful scientist΄s "friend".
Figures
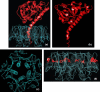

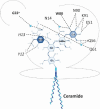
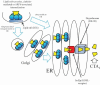

References
-
- De SN. Enterotoxicity of bacteria-free culture-filtrate of Vibrio cholerae. Nature. 1959;183:1533–4. - PubMed
-
- Lonnroth I, Holmgren J. Subunit structure of cholera toxin. J Gen Microbiol. 1973;76:417–27. - PubMed
-
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981;292:413–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
