Phospholipase A(2) of peroxiredoxin 6 has a critical role in tumor necrosis factor-induced apoptosis
- PMID: 21415860
- PMCID: PMC3172113
- DOI: 10.1038/cdd.2011.21
Phospholipase A(2) of peroxiredoxin 6 has a critical role in tumor necrosis factor-induced apoptosis
Abstract
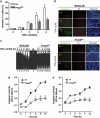
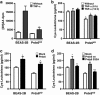
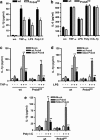
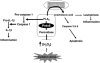
Peroxiredoxin 6 (Prdx6) is a bifunctional enzyme with peroxidase and phospholipase A(2) (PLA(2)) activities. Although the cellular function of the peroxidase of Prdx6 has been well elucidated, the function of the PLA(2) of Prdx6 is largely unknown. Here, we report a novel function for the PLA(2) in regulating TNF-induced apoptosis through arachidonic acid (AA) release and interleukin-1β (IL-1β) production. Prdx6 knockdown (Prdx6(KD)) in human bronchial epithelial cells (BEAS2B) shows severe decreases of peroxidase and PLA(2) activities. Surprisingly, Prdx6(KD) cells are markedly resistant to apoptosis induced by TNF-α in the presence of cycloheximide, but are highly sensitive to hydrogen peroxide-induced apoptosis. Furthermore, the release of AA and the production of IL-1β induced by proinflammatory stimuli, such as TNF-α, LPS, and poly I/C, are severely decreased in Prdx6(KD) cells. More interestingly, the restoration of Prdx6 expression with wild-type Prdx6, but not PLA(2)-mutant Prdx6 (S32A), in Prdx6(KD) cells dramatically induces the recovery of TNF-induced apoptosis, AA release, and IL-1β production, indicating specific roles for the PLA(2) activity of Prdx6. Our results provide new insights into the distinct roles of bifunctional Prdx6 with peroxidase and PLA(2) activities in oxidative stress-induced and TNF-induced apoptosis, respectively.
Figures




Similar articles
-
Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages.J Biol Chem. 2011 Apr 1;286(13):11696-706. doi: 10.1074/jbc.M110.206623. Epub 2011 Jan 24. J Biol Chem. 2011. PMID: 21262967 Free PMC article.
-
The roles of peroxidase and phospholipase A2 activities of peroxiredoxin 6 in protecting pulmonary microvascular endothelial cells against peroxidative stress.Antioxid Redox Signal. 2012 Mar 1;16(5):440-51. doi: 10.1089/ars.2011.3950. Epub 2011 Dec 23. Antioxid Redox Signal. 2012. PMID: 22067043 Free PMC article.
-
Phospholipase A2 activity of peroxiredoxin 6 promotes invasion and metastasis of lung cancer cells.Mol Cancer Ther. 2010 Apr;9(4):825-32. doi: 10.1158/1535-7163.MCT-09-0904. Epub 2010 Mar 30. Mol Cancer Ther. 2010. PMID: 20354123
-
Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A₂ activities.Antioxid Redox Signal. 2011 Aug 1;15(3):831-44. doi: 10.1089/ars.2010.3412. Epub 2011 Mar 31. Antioxid Redox Signal. 2011. PMID: 20919932 Free PMC article. Review.
-
Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism.Free Radic Biol Med. 2005 Jun 1;38(11):1422-32. doi: 10.1016/j.freeradbiomed.2005.02.011. Free Radic Biol Med. 2005. PMID: 15890616 Review.
Cited by
-
Knockout of PRDX6 induces mitochondrial dysfunction and cell cycle arrest at G2/M in HepG2 hepatocarcinoma cells.Redox Biol. 2020 Oct;37:101737. doi: 10.1016/j.redox.2020.101737. Epub 2020 Sep 29. Redox Biol. 2020. PMID: 33035814 Free PMC article.
-
The Role of Peroxiredoxin 6 in Cell Signaling.Antioxidants (Basel). 2018 Nov 24;7(12):172. doi: 10.3390/antiox7120172. Antioxidants (Basel). 2018. PMID: 30477202 Free PMC article. Review.
-
Inactivation of Peroxiredoxin 6 by the Pla Protease of Yersinia pestis.Infect Immun. 2015 Nov 9;84(1):365-74. doi: 10.1128/IAI.01168-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26553463 Free PMC article.
-
AMP-activated protein kinase-α1 as an activating kinase of TGF-β-activated kinase 1 has a key role in inflammatory signals.Cell Death Dis. 2012 Jul 26;3(7):e357. doi: 10.1038/cddis.2012.95. Cell Death Dis. 2012. PMID: 22833096 Free PMC article.
-
Tuning of peroxiredoxin catalysis for various physiological roles.Biochemistry. 2014 Dec 16;53(49):7693-705. doi: 10.1021/bi5013222. Epub 2014 Dec 1. Biochemistry. 2014. PMID: 25403613 Free PMC article. Review.
References
-
- Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41. - PubMed
-
- Woo HA, Chae HZ, Hwang SC, Yang KS, Kang SW, Kim K, et al. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–656. - PubMed
-
- Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. - PubMed
-
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. - PubMed
-
- Chen JW, Dodia C, Feinstein SI, Jain MK, Fisher AB. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 Activities. J Biol Chem. 2000;275:28421–28427. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

