Evaluation of a novel supplement to reduce blood glucose through the use of a modified oral glucose tolerance test
- PMID: 21416063
- PMCID: PMC3056567
Evaluation of a novel supplement to reduce blood glucose through the use of a modified oral glucose tolerance test
Abstract
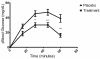
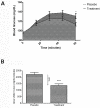
Elevated blood glucose is a major component in metabolic syndrome and pre-diabetes, sometimes leading to type 2 diabetes mellitus (DM II). Additionally, it may lead to adipose deposits when left elevated for long periods. The epidemiology of DM II clearly shows that uncontrolled blood glucose levels leads to many adverse conditions including heart disease, retinal damage, renal failure, erectile dysfunction, and other significant medical conditions. Here we conducted a single-center, prospective, randomized, double-blinded, placebo-controlled, parallel-group- clinical trial of a nutraceutical supplement vs. placebo to measure its glucose lowering effect in generally healthy adults before and after a simple sugars meal. Subjects reported to the test clinic on multiple days to receive placebo or treatment, a simple sugars meal, as well as pre-and postprandial blood glucose measurement (modified oral glucose tolerance test). Each subject served as his or her own control and thirty-one subjects completed the trial with at least one oral glucose tolerance test (OGTT) with the nutraceutical supplement and placebo. Statistical analysis revealed the nutraceutical supplement significantly lowered postprandial glucose levels by 36% and 59% at 45 and 60 minutes, respectively (***P<.001). The study was limited by its composition of primarily overweight females. Future studies will be required over longer periods in more heterogeneous and larger groups to determine the long-term effect of this supplement on blood glucose levels in terms of prophylaxis or treatment for DM II.
Keywords: Glucose tolerance test; diabetes mellitus II; diet; hyperglycemia; insulin resistance; nutraceutical supplement.
Figures
Similar articles
-
Excess glycaemic excursions after an oral glucose tolerance test compared with a mixed meal challenge and self-measured home glucose profiles: is the OGTT a valid predictor of postprandial hyperglycaemia and vice versa?Diabetes Obes Metab. 2009 Mar;11(3):213-22. doi: 10.1111/j.1463-1326.2008.00922.x. Epub 2008 Jun 16. Diabetes Obes Metab. 2009. PMID: 18564177
-
Subcutaneous microdialysis before and after an oral glucose tolerance test: a method to determine insulin resistance in the subcutaneous adipose tissue in diabetes mellitus.Diabetes Obes Metab. 2005 Sep;7(5):525-35. doi: 10.1111/j.1463-1326.2004.00424.x. Diabetes Obes Metab. 2005. PMID: 16050945
-
Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals.J Physiol. 2018 Apr 15;596(8):1385-1395. doi: 10.1113/JP275709. Epub 2018 Mar 2. J Physiol. 2018. PMID: 29446830 Free PMC article. Clinical Trial.
-
Repaglinide : a pharmacoeconomic review of its use in type 2 diabetes mellitus.Pharmacoeconomics. 2004;22(6):389-411. doi: 10.2165/00019053-200422060-00005. Pharmacoeconomics. 2004. PMID: 15099124 Review.
-
[Gestational diabetes mellitus (Update 2019)].Wien Klin Wochenschr. 2019 May;131(Suppl 1):91-102. doi: 10.1007/s00508-018-1419-8. Wien Klin Wochenschr. 2019. PMID: 30980150 Review. German.
References
-
- Balch JF, Balch PA. Garden City Park, N.Y.: Avery Pub. Group; 1997. Prescription for nutritional healing.
-
- McCarty MF. Nutraceutical resources for diabetes prevention–an update. Med Hypotheses. 2005;64:151–158. - PubMed
-
- Vuksan V, Sievenpiper JL, Koo VY, Francis T, Beljan-Zdravkovic U, Xu Z, Vidgen E. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med. 2000;160:1009–1013. - PubMed
-
- Roberts AJ, O'Brien ME, Subak-Sharpe GJ. New York: Perigee Book; 2001. Nutraceuticals : the complete encyclopedia of supplements, herbs, vitamins, and healing foods.
Grants and funding
LinkOut - more resources
Full Text Sources
