Intertwined αβ spectrin meeting helical actin protofilament in the erythrocyte membrane skeleton: wrap-around vs. point-attachment
- PMID: 21416170
- PMCID: PMC3110870
- DOI: 10.1007/s10439-011-0293-6
Intertwined αβ spectrin meeting helical actin protofilament in the erythrocyte membrane skeleton: wrap-around vs. point-attachment
Abstract
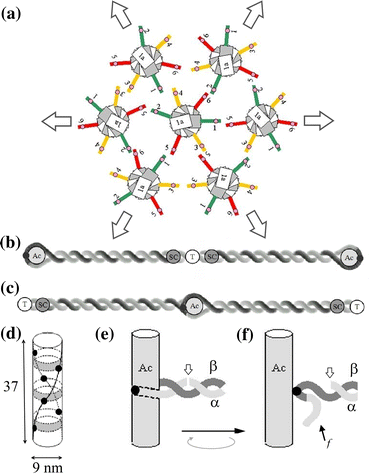
Our 3-D model for a junctional complex (JC) in the erythrocyte membrane skeleton proposed that the helical actin protofilament functions as a mechanical axis for three pairs of αβ spectrin (Sp), and each pair wraps around the protofilament in a back-to-back fashion. The distal end of each Sp is further associated with the lipid bilayer by a suspension complex (SC). Here, we detail how splitting and rejoining of αβ Sp around a protofilament may form a loop that sustains and equilibrates tension. Sequential association of β and α Sp solves the challenge of constructing multiple loops along the protofilament, and topological connection facilitates their re-association. The wrap-around model minimizes the strain of the actin binding site on β Sp due to tension, redirection, or sliding of intertwined Sp. Pairing Sp balances the opposing forces and provides a mechanism for elastic recovery. The wrap-around junction thus provides mechanical advantages over a point-attachment junction in maintaining the integrity and functionality of the network. Severing α or β Sp may convert a wrapping-around junction to a point-attachment junction. In that case, a "bow up" motion of JC during deformation may disturb or flip the overlaid lipid bilayer, and mark stressed erythrocytes for phagocytosis.
Figures
Similar articles
-
Protofilament and hexagon: a three-dimensional mechanical model for the junctional complex in the erythrocyte membrane skeleton.Ann Biomed Eng. 2003 Dec;31(11):1314-26. doi: 10.1114/1.1635820. Ann Biomed Eng. 2003. PMID: 14758922
-
A hybrid model for erythrocyte membrane: a single unit of protein network coupled with lipid bilayer.Biophys J. 2007 Jul 15;93(2):386-400. doi: 10.1529/biophysj.106.094383. Epub 2007 Apr 20. Biophys J. 2007. PMID: 17449663 Free PMC article.
-
3-D nanomechanics of an erythrocyte junctional complex in equibiaxial and anisotropic deformations.Ann Biomed Eng. 2005 Oct;33(10):1387-404. doi: 10.1007/s10439-005-4698-y. Ann Biomed Eng. 2005. PMID: 16240087
-
Anatomy of the red cell membrane skeleton: unanswered questions.Blood. 2016 Jan 14;127(2):187-99. doi: 10.1182/blood-2014-12-512772. Epub 2015 Nov 4. Blood. 2016. PMID: 26537302 Review.
-
The spectrin-actin junction of erythrocyte membrane skeletons.Biochim Biophys Acta. 1989 Jan 18;988(1):107-21. doi: 10.1016/0304-4157(89)90006-3. Biochim Biophys Acta. 1989. PMID: 2642392 Review.
References
-
- Bratosin D, Mazurier J, Tissier JP, Slomianny C, Estaquier J, Russo-Marie F, Huart JJ, Freyssinet JM, Aminoff D, Ameisen JC, et al. Molecular mechanisms of erythrophagocytosis. Characterization of the senescent erythrocytes that are phagocytized by macrophages. C. R. Acad. Sci. III. 1997;320(10):811–818. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

