Stereoelectronic factors in the stereoselective epoxidation of glycals and 4-deoxypentenosides
- PMID: 21417287
- PMCID: PMC3074037
- DOI: 10.1021/jo102382r
Stereoelectronic factors in the stereoselective epoxidation of glycals and 4-deoxypentenosides
Abstract
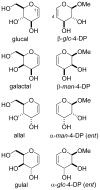
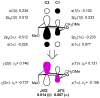
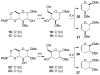
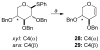
Glycals and 4-deoxypentenosides (4-DPs), unsaturated pyranosides with similar structures and reactivity profiles, can exhibit a high degree of stereoselectivity upon epoxidation with dimethyldioxirane (DMDO). In most cases, the glycals and their corresponding 4-DP isosteres share the same facioselectivity, implying that the pyran substituents are largely responsible for the stereodirecting effect. Fully substituted dihydropyrans are subject to a "majority rule", in which the epoxidation is directed toward the face opposite to two of the three groups. Removing one of the substituents has a variable effect on the epoxidation outcome, depending on its position and also on the relative stereochemistry of the remaining two groups. Overall, we observe that the greatest loss in facioselectivity for glycals and 4-DPs is caused by removal of the C3 oxygen, followed by the C5/anomeric substituent, and least of all by the C4/C2 oxygen. DFT calculations based on polarized-π frontier molecular orbital (PPFMO) theory support a stereoelectronic role for the oxygen substituents in 4-DP facioselectivity, but less clearly so in the case of glycals. We conclude that the anomeric oxygen in 4-DPs contributes toward a stereoelectronic bias in facioselectivity whereas the C5 alkoxymethyl in glycals imparts a steric bias, which at times can compete with the stereodirecting effects from the other oxygen substituents.
Figures



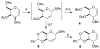

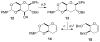
Similar articles
-
Stereoselective epoxidation of 4-deoxypentenosides: a polarized-pi model.Org Lett. 2006 Sep 28;8(20):4545-8. doi: 10.1021/ol0617401. Org Lett. 2006. PMID: 16986946 Free PMC article.
-
Synthesis of L-sugars from 4-deoxypentenosides.Org Lett. 2002 Jun 27;4(13):2281-3. doi: 10.1021/ol026175q. Org Lett. 2002. PMID: 12074687
-
Epoxidation of C-branched glycals: unexpected stereochemical results and their theoretical rationale.Carbohydr Res. 2003 Jan 31;338(3):231-6. doi: 10.1016/s0008-6215(02)00406-8. Carbohydr Res. 2003. PMID: 12543555
-
[Stereoselective epoxidation with bulky dioxiranes generated from substituted cyclohexanones].Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 1998;(116):63-8. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 1998. PMID: 10097513 Review. Japanese.
-
Stereoelectronic substituent effects.Acc Chem Res. 2006 Apr;39(4):259-65. doi: 10.1021/ar050189p. Acc Chem Res. 2006. PMID: 16618093 Review.
Cited by
-
Glycal assembly by the in situ generation of glycosyl dithiocarbamates.Org Lett. 2012 Jul 6;14(13):3380-3. doi: 10.1021/ol301349w. Epub 2012 Jun 11. Org Lett. 2012. PMID: 22686424 Free PMC article.
-
Glycal Metallanitrenes for 2-Amino Sugar Synthesis: Amidoglycosylation of Gulal-, Allal-, Glucal-, and Galactal 3-Carbamates.J Org Chem. 2018 Aug 3;83(15):8054-8080. doi: 10.1021/acs.joc.8b00893. Epub 2018 Jul 6. J Org Chem. 2018. PMID: 29979042 Free PMC article.
-
Synthesis and reactivity of 4'-deoxypentenosyl disaccharides.J Org Chem. 2014 Jun 6;79(11):4878-91. doi: 10.1021/jo500449h. Epub 2014 May 12. J Org Chem. 2014. PMID: 24797640 Free PMC article.
-
Turning Spiroketals Inside Out: A Rearrangement Triggered by an Enol Ether Epoxidation.ChemistryOpen. 2015 Oct;4(5):577-80. doi: 10.1002/open.201500122. Epub 2015 Jun 17. ChemistryOpen. 2015. PMID: 26491634 Free PMC article.
-
Creation of a macrolide antibiotic against non-tuberculous Mycobacterium using late-stage boron-mediated aglycon delivery.Sci Adv. 2025 Mar 7;11(10):eadt2352. doi: 10.1126/sciadv.adt2352. Epub 2025 Mar 5. Sci Adv. 2025. PMID: 40043128 Free PMC article.
References
-
- Tolstikov AG, Tolstikov GA. Russ Chem Rev. 1993;62(6):579–601.
- Ferrier RJ, Hoberg JO. In: Advances in Carbohydrate Chemistry and Biochemistry. Horton D, editor. Vol. 58. Academic Press; New York: 2003. pp. 55–119. - PubMed
-
- Danishefsky SJ, Bilodeau MT. Angew Chem Int Ed Engl. 1996;35:1380–1419.
- Plante OJ, Andrade RB, Seeberger PH. Org Lett. 1999;1:211–214. - PubMed
- Cheshev P, Marra A, Dondoni A. Carbohydr Res. 2006;341:2714–2716. - PubMed
- Koester DC, Leibeling M, Neufeld R, Werz DB. Org Lett. 2010;12:3934–3937. - PubMed
-
-
Examples of nonpyranoid glycals: Singh I, Seitz O. Org Lett. 2006;8:4319–4322.Markad S, Xia S, Surana B, Morton MD, Hadad CM, Peczuh MW. J Org Chem. 2008;73:6341–6354.Saha J, Peczuh MW. Org Lett. 2009;11:4482–4484.
-
-
- Brigl P. Hoppe-Seyl Z Physiol Chem. 1921;116:1.
-
- Halcomb RL, Danishefsky SJ. J Am Chem Soc. 1989;111:6661–6666.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

