Transgenic expression of human S100A12 induces structural airway abnormalities and limited lung inflammation in a mouse model of allergic inflammation
- PMID: 21418345
- PMCID: PMC3093439
- DOI: 10.1111/j.1365-2222.2011.03714.x
Transgenic expression of human S100A12 induces structural airway abnormalities and limited lung inflammation in a mouse model of allergic inflammation
Abstract
Background: The calcium-binding protein S100A12 is highly up-regulated in the serum and sputum of patients with allergic asthma and is suggested to be a biomarker and pathologic mediator of asthma.
Objective: To test the role of S100A12 in mediating airway inflammation in a mouse model of allergic lung inflammation.
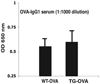
Methods: Transgenic (TG) mice that express human S100A12 and wild-type (WT) littermates were sensitized and challenged with ovalbumin (OVA) and assessed for inflammation, lung structure, and function.
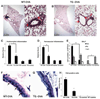
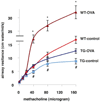
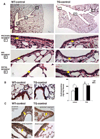
Results: Following OVA sensitization and challenge, S100A12 TG mice showed reduced peribronchial and perivascular inflammation, mucus production, and eosinophilia as well as attenuated airway responsiveness to contractile agonist compared with WT sensitized and challenged animals. This is explained, at least in part, by remodelled airways in S100A12 TG mice with thinning of the airway smooth muscle. S100A12 exposure induced Fas expression and activation of caspase 3 in cultured airway smooth muscle cells, suggesting that airway smooth muscle abnormalities observed in S100A12 TG mice may be mediated through myocyte apoptosis.
Conclusion and clinical relevance: S100A12 is one of the most abundant proteins found in the airways of human asthmatics, and it was postulated that S100A12 could mediate the inflammatory process. Our study shows for the first time that TG expression of S100A12 in the lung of mice does not exacerbate lung inflammation in a model of OVA-induced allergic inflammation. We speculate that the high levels of S100/calgranulins found in bronchoalveolar lavage fluid of asthmatics and of OVA-treated TG S100A12 mice do not significantly mediate pulmonary inflammation.
© 2011 Blackwell Publishing Ltd.
Conflict of interest statement
The authors have no financial conflict of interest. Disclosure: MA. Hofmann Bowman, None; A. Heydeman, None; J. Gawdzik, None; R. Shilling, None; B. Camoretti-Mercado, None.
Figures

Similar articles
-
Mice deficient in the St3gal3 gene product α2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation.J Allergy Clin Immunol. 2014 Jan;133(1):240-7.e1-3. doi: 10.1016/j.jaci.2013.05.018. Epub 2013 Jul 2. J Allergy Clin Immunol. 2014. PMID: 23830412 Free PMC article.
-
Protein tyrosine phosphatase SHP2 regulates TGF-β1 production in airway epithelia and asthmatic airway remodeling in mice.Allergy. 2012 Dec;67(12):1547-56. doi: 10.1111/all.12048. Epub 2012 Oct 11. Allergy. 2012. PMID: 23057634 Free PMC article.
-
M(3) muscarinic receptor antagonist bencycloquidium bromide attenuates allergic airway inflammation, hyperresponsiveness and remodeling in mice.Eur J Pharmacol. 2011 Mar 25;655(1-3):83-90. doi: 10.1016/j.ejphar.2011.01.024. Epub 2011 Jan 28. Eur J Pharmacol. 2011. PMID: 21277298
-
Platelets are necessary for airway wall remodeling in a murine model of chronic allergic inflammation.Blood. 2004 Jan 15;103(2):639-47. doi: 10.1182/blood-2003-05-1707. Epub 2003 Sep 22. Blood. 2004. PMID: 14504080
-
Allergen-induced airway remodeling is impaired in galectin-3-deficient mice.J Immunol. 2010 Jul 15;185(2):1205-14. doi: 10.4049/jimmunol.1000039. Epub 2010 Jun 11. J Immunol. 2010. PMID: 20543100 Free PMC article.
Cited by
-
Functions of S100 proteins.Curr Mol Med. 2013 Jan;13(1):24-57. Curr Mol Med. 2013. PMID: 22834835 Free PMC article. Review.
-
S100A12 suppresses pro-inflammatory, but not pro-thrombotic functions of serum amyloid A.PLoS One. 2013 Apr 24;8(4):e62372. doi: 10.1371/journal.pone.0062372. Print 2013. PLoS One. 2013. PMID: 23638054 Free PMC article.
-
Emerging roles of neutrophil-borne S100A8/A9 in cardiovascular inflammation.Pharmacol Res. 2020 Nov;161:105212. doi: 10.1016/j.phrs.2020.105212. Epub 2020 Sep 28. Pharmacol Res. 2020. PMID: 32991974 Free PMC article. Review.
-
Diet Transition from High-Forage to High-Concentrate Alters Rumen Bacterial Community Composition, Epithelial Transcriptomes and Ruminal Fermentation Parameters in Dairy Cows.Animals (Basel). 2021 Mar 16;11(3):838. doi: 10.3390/ani11030838. Animals (Basel). 2021. PMID: 33809588 Free PMC article.
-
S100A12 expression in thoracic aortic aneurysm is associated with increased risk of dissection and perioperative complications.J Am Coll Cardiol. 2012 Aug 21;60(8):775-85. doi: 10.1016/j.jacc.2012.04.027. Epub 2012 Jul 18. J Am Coll Cardiol. 2012. PMID: 22818064 Free PMC article.
References
-
- Wu J, Kobayashi M, Sousa EA, Liu W, Cai J, Goldman SJ, Dorner AJ, Projan SJ, Kavuru MS, Qiu Y, Thomassen MJ. Differential proteomic analysis of bronchoalveolar lavage fluid in asthmatics following segmental antigen challenge. Mol Cell Proteomics. 2005;4:1251–1264. - PubMed
-
- Heizmann CW, Ackermann GE, Galichet A. Pathologies involving the S100 proteins and RAGE. Subcell Biochem. 2007;45:93–138. - PubMed
-
- Foell D, Kane D, Bresnihan B, Vogl T, Nacken W, Sorg C, Fitzgerald O, Roth J. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology (Oxford) 2003;42:1383–1389. - PubMed
-
- Foell D, Ichida F, Vogl T, Yu X, Chen R, Miyawaki T, Sorg C, Roth J. S100A12 (EN-RAGE) in monitoring Kawasaki disease. Lancet. 2003;361:1270–1272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

