Nav2 hypomorphic mutant mice are ataxic and exhibit abnormalities in cerebellar development
- PMID: 21419114
- PMCID: PMC3250223
- DOI: 10.1016/j.ydbio.2011.03.008
Nav2 hypomorphic mutant mice are ataxic and exhibit abnormalities in cerebellar development
Abstract
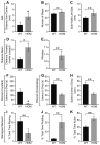
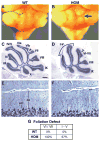
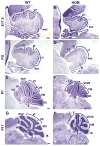
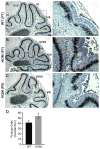
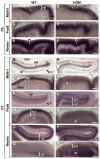
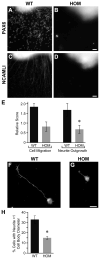
Development of the cerebellum involves a coordinated program of neuronal process outgrowth and migration resulting in a foliated structure that plays a key role in motor function. Neuron navigator 2 (Nav2) is a cytoskeletal-interacting protein that functions in neurite outgrowth and axonal elongation. Herein we show that hypomorphic mutant mice lacking the full-length Nav2 transcript exhibit ataxia and defects in cerebellar development. At embryonic day (E)17.5, the mutant cerebellum is reduced in size and exhibits defects in vermal foliation. Reduction in cell proliferation at early times (E12.5 and E14.5) may contribute to this size reduction. The full-length Nav2 transcript is expressed in the premigratory zone of the external granule layer (EGL). Granule cells in the germinal zone of the EGL appear to proliferate normally, however, due to the reduction in cerebellar circumference there are fewer total BrdU-labeled granule cells in the mutants, and these fail to migrate normally toward the interior of the cerebellum. In Nav2 hypomorphs, fewer granule cells migrate out of cerebellar EGL explants and neurite outgrowth from both explants and isolated external granule cell cultures is reduced. This suggests that the formation of parallel axon fibers and neuronal migration is disrupted in Nav2 mutants. This work supports an essential role for full-length Nav2 in cerebellar development, including axonal elongation and migration of the EGL neurons.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Expression pattern of Nav2 in the murine CNS with development.Gene Expr Patterns. 2020 Jan;35:119099. doi: 10.1016/j.gep.2020.119099. Epub 2020 Feb 18. Gene Expr Patterns. 2020. PMID: 32081718
-
Persistent motor dysfunction despite homeostatic rescue of cerebellar morphogenesis in the Car8 waddles mutant mouse.Neural Dev. 2019 Mar 12;14(1):6. doi: 10.1186/s13064-019-0130-4. Neural Dev. 2019. PMID: 30867000 Free PMC article.
-
Loss of Neuron Navigator 2 Impairs Brain and Cerebellar Development.Cerebellum. 2023 Apr;22(2):206-222. doi: 10.1007/s12311-022-01379-3. Epub 2022 Feb 26. Cerebellum. 2023. PMID: 35218524 Free PMC article.
-
Zebrin II expressing Purkinje cell phenotype-related and -unrelated cerebellar abnormalities in Cav2.1 mutant, rolling mouse Nagoya.ScientificWorldJournal. 2010 Oct 12;10:2032-8. doi: 10.1100/tsw.2010.205. ScientificWorldJournal. 2010. PMID: 20953553 Free PMC article. Review.
-
Can clues from evolution unlock the molecular development of the cerebellum?Mol Neurobiol. 2011 Feb;43(1):67-76. doi: 10.1007/s12035-010-8160-2. Epub 2010 Dec 21. Mol Neurobiol. 2011. PMID: 21174175 Review.
Cited by
-
Discovery of a Family of Genomic Sequences Which Interact Specifically with the c-MYC Promoter to Regulate c-MYC Expression.PLoS One. 2016 Aug 23;11(8):e0161588. doi: 10.1371/journal.pone.0161588. eCollection 2016. PLoS One. 2016. PMID: 27551915 Free PMC article.
-
The Neuron Navigators: Structure, function, and evolutionary history.Front Mol Neurosci. 2023 Jan 12;15:1099554. doi: 10.3389/fnmol.2022.1099554. eCollection 2022. Front Mol Neurosci. 2023. PMID: 36710926 Free PMC article. Review.
-
CPEB3 regulates neuron-specific alternative splicing and involves neurogenesis gene expression.Aging (Albany NY). 2020 Dec 9;13(2):2330-2347. doi: 10.18632/aging.202259. Epub 2020 Dec 9. Aging (Albany NY). 2020. PMID: 33318303 Free PMC article.
-
The +TIP Navigator-1 is an actin-microtubule crosslinker that regulates axonal growth cone motility.J Cell Biol. 2020 Sep 7;219(9):e201905199. doi: 10.1083/jcb.201905199. J Cell Biol. 2020. PMID: 32497170 Free PMC article.
-
Mutated neuron navigator 3 as a candidate gene for a rare neurodevelopmental disorder.Mol Genet Genomic Med. 2024 Jul;12(7):e2473. doi: 10.1002/mgg3.2473. Mol Genet Genomic Med. 2024. PMID: 39038237 Free PMC article.
References
-
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev. 2008;9:309–322. - PubMed
-
- Bancroft JD, Stevens A. Theory and practice of histological techniques. 3. Churchill Livingstone; London: 1990.
-
- Cajal SR. Texture of the nervous system of man and the vertebrates. Springer-Verlag/Wien; New York: 1911. 2000.
-
- Chédotal A. Should I stay or should I go? Becoming a granule cell. Trends Neurosci. 2010;33:163–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

