Demasculinization and feminization of male gonads by atrazine: consistent effects across vertebrate classes
- PMID: 21419222
- PMCID: PMC4303243
- DOI: 10.1016/j.jsbmb.2011.03.015
Demasculinization and feminization of male gonads by atrazine: consistent effects across vertebrate classes
Abstract
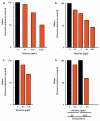
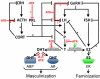
Atrazine is the most commonly detected pesticide contaminant of ground water, surface water, and precipitation. Atrazine is also an endocrine disruptor that, among other effects, alters male reproductive tissues when animals are exposed during development. Here, we apply the nine so-called "Hill criteria" (Strength, Consistency, Specificity, Temporality, Biological Gradient, Plausibility, Coherence, Experiment, and Analogy) for establishing cause-effect relationships to examine the evidence for atrazine as an endocrine disruptor that demasculinizes and feminizes the gonads of male vertebrates. We present experimental evidence that the effects of atrazine on male development are consistent across all vertebrate classes examined and we present a state of the art summary of the mechanisms by which atrazine acts as an endocrine disruptor to produce these effects. Atrazine demasculinizes male gonads producing testicular lesions associated with reduced germ cell numbers in teleost fish, amphibians, reptiles, and mammals, and induces partial and/or complete feminization in fish, amphibians, and reptiles. These effects are strong (statistically significant), consistent across vertebrate classes, and specific. Reductions in androgen levels and the induction of estrogen synthesis - demonstrated in fish, amphibians, reptiles, and mammals - represent plausible and coherent mechanisms that explain these effects. Biological gradients are observed in several of the cited studies, although threshold doses and patterns vary among species. Given that the effects on the male gonads described in all of these experimental studies occurred only after atrazine exposure, temporality is also met here. Thus the case for atrazine as an endocrine disruptor that demasculinizes and feminizes male vertebrates meets all nine of the "Hill criteria".
Copyright © 2011. Published by Elsevier Ltd.
Figures


References
-
- Solomon K, et al. Ecological risk assessment of atrazine in North American surface waters. Environ. Toxicol. Chem. 1996;15:31–76. - PubMed
-
- Boyd R. Herbicides and herbicide degradates in shallow groundwater and the Cedar River near a municipal well field, Cedar Rapids, Iowa, Sci. Total Environ. 2000;248:241–253. - PubMed
-
- Capel P, Larson S. Effect of scale on the behavior of atrazine in surface waters. Environ. Sci. Technol. 2001;35(4):648–657. - PubMed
-
- Du Preez LH, et al. Seasonal exposures to triazine and other pesticides in surface waters in the western Highveld corn-production region in South Africa. Environ. Pollut. 2005;135(1):131–141. - PubMed
-
- Fenelon J, Moore R. Transport of agrichemicals to ground and surface waters in a small central Indiana watershed. J. Environ. Qual. 1998;27:884–894.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

