A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range
- PMID: 21419439
- PMCID: PMC3140702
- DOI: 10.1016/j.juro.2010.12.032
A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range
Erratum in
- J Urol. 2011 Jul;186(1):354
Abstract
Purpose: Prostate specific antigen and free prostate specific antigen have limited specificity to detect clinically significant, curable prostate cancer, leading to unnecessary biopsy, and detection and treatment of some indolent tumors. Specificity to detect clinically significant prostate cancer may be improved by [-2]pro-prostate specific antigen. We evaluated [-2]pro-prostate specific antigen, free prostate specific antigen and prostate specific antigen using the formula, ([-2]pro-prostate specific antigen/free prostate specific antigen × prostate specific antigen(1/2)) to enhance specificity to detect overall and high grade prostate cancer.
Materials and methods: We enrolled 892 men with no history of prostate cancer, normal rectal examination, prostate specific antigen 2 to 10 ng/ml and 6-core or greater prostate biopsy in a prospective multi-institutional trial. We examined the relationship of serum prostate specific antigen, free-to-total prostate specific antigen and the prostate health index with biopsy results. Primary end points were specificity and AUC using the prostate health index to detect overall and Gleason 7 or greater prostate cancer on biopsy compared with those of free-to-total prostate specific antigen.
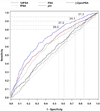
Results: In the 2 to 10 ng/ml prostate specific antigen range at 80% to 95% sensitivity the specificity and AUC (0.703) of the prostate health index exceeded those of prostate specific antigen and free-to-total prostate specific antigen. An increasing prostate health index was associated with a 4.7-fold increased risk of prostate cancer and a 1.61-fold increased risk of Gleason score greater than or equal to 4 + 3 = 7 disease on biopsy. The AUC of the index exceeded that of free-to-total prostate specific antigen (0.724 vs 0.670) to discriminate prostate cancer with Gleason 4 or greater + 3 from lower grade disease or negative biopsy. Prostate health index results were not associated with age and prostate volume.
Conclusions: The prostate health index may be useful in prostate cancer screening to decrease unnecessary biopsy in men 50 years old or older with prostate specific antigen 2 to 10 ng/ml and negative digital rectal examination with minimal loss in sensitivity.
Copyright © 2011 American Urological Association Education and Research, Inc. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Krumholtz JS, Carvalhal GF, Ramos CG, et al. Prostate-specific antigen cutoff of 2.6 ng/mL for prostate cancer screening is associated with favorable pathologic tumor features. Urology. 2002;60:469. - PubMed
-
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239. - PubMed
-
- Catalona WJ, Richie JP, Ahmann FR, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283. - PubMed
-
- Lilja H, Christensson A, Dahlen U, et al. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin Chem. 1991;37:1618. - PubMed
-
- Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. Jama. 1998;279:1542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA90386-05S2/CA/NCI NIH HHS/United States
- CA091956/CA/NCI NIH HHS/United States
- P50 CA058236/CA/NCI NIH HHS/United States
- P30 CA006973/CA/NCI NIH HHS/United States
- P50CA58236/CA/NCI NIH HHS/United States
- UL1 RR025741/RR/NCRR NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- U01-CA86323/CA/NCI NIH HHS/United States
- U01 CA113913/CA/NCI NIH HHS/United States
- U01CA113913/CA/NCI NIH HHS/United States
- P50 CA091956/CA/NCI NIH HHS/United States
- U01 CA086323/CA/NCI NIH HHS/United States
- U24 CA115102/CA/NCI NIH HHS/United States
- P50 CA090386/CA/NCI NIH HHS/United States
- P30 CA60553/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical