The collateral network concept: remodeling of the arterial collateral network after experimental segmental artery sacrifice
- PMID: 21419904
- PMCID: PMC3086022
- DOI: 10.1016/j.jtcvs.2010.06.017
The collateral network concept: remodeling of the arterial collateral network after experimental segmental artery sacrifice
Abstract
Objective: A comprehensive strategy to prevent paraplegia after open surgical or endovascular repair of thoracoabdominal aortic aneurysms requires a thorough understanding of the response of the collateral network to extensive segmental artery sacrifice.
Methods: Ten Yorkshire pigs underwent perfusion with a low-viscosity acrylic resin. With the use of cardiopulmonary bypass, 2 animals each were perfused in the native state and immediately, 6 hours, 24 hours, and 5 days after sacrifice of all segmental arteries (T4-L5). After digestion of surrounding tissue, the vascular cast of the collateral network underwent analysis of arterial and arteriolar diameters and the density and spatial orientation of the vasculature using light and scanning electron microscopy.
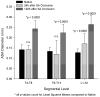
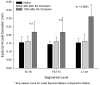
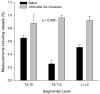
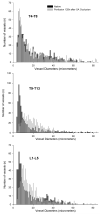
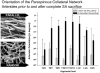
Results: Within 24 hours, the diameter of the anterior spinal artery had increased significantly, and within 5 days the anterior spinal artery and the epidural arterial network had enlarged in diameter by 80% to 100% (P < .0001). By 5 days, the density of the intramuscular paraspinous vessels had increased (P < .0001), a shift of size distribution from small to larger arterioles was seen (P = .0002), and a significant realignment of arterioles parallel to the spinal cord had occurred (P = .0005).
Conclusions: Within 5 days after segmental artery occlusion, profound anatomic alterations in the intraspinal and paraspinous arteries and arterioles occurred, providing the anatomic substrate for preservation of spinal cord blood flow via collateral pathways.
Copyright © 2011 The American Association for Thoracic Surgery. Published by Mosby, Inc. All rights reserved.
Figures



References
-
- Maniar HS, Sundt TM, 3rd, Prasad SM, Chu CM, Camillo CJ, Moon MR, et al. Delayed paraplegia after thoracic and thoracoabdominal aneurysm repair: a continuing risk. Ann Thorac Surg. 2003;75(1):113–9. discussions 19-20. - PubMed
-
- Etz CD, Luehr M, Kari FA, Bodian CA, Smego D, Plestis KA, et al. Paraplegia after extensive thoracic and thoracoabdominal aortic aneurysm repair: does critical spinal cord ischemia occur postoperatively? J Thorac Cardiovasc Surg. 2008;135(2):324–30. - PubMed
-
- Etz CD, Halstead JC, Spielvogel D, Shahani R, Lazala R, Homann TM, et al. Thoracic and thoracoabdominal aneurysm repair: is reimplantation of spinal cord arteries a waste of time? Ann Thorac Surg. 2006;82(5):1670–7. - PubMed
-
- Etz CD, Di Luozzo G, Zoli S, Lazala R, Plestis KA, Bodian CA, et al. Direct spinal cord perfusion pressure monitoring in extensive distal aortic aneurysm repair. Ann Thorac Surg. 2009;87(6):1764–73. discussion 73-4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

