Smad1 plays an essential role in bone development and postnatal bone formation
- PMID: 21420501
- PMCID: PMC3113680
- DOI: 10.1016/j.joca.2011.03.004
Smad1 plays an essential role in bone development and postnatal bone formation
Abstract
Objectives: To determine the role of Smad1 in bone development and postnatal bone formation.
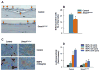
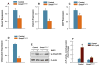
Methods: Col2a1-Cre transgenic mice were bred with Smad1(fx/fx) mice to produce chondrocyte-specific Smad1 conditional knockout (cKO) mice. Embryonic skeletal preparation and staining were performed, alkaline phosphatase activity (ALP) and relative gene expression were examined in isolated primary cells. Smad1(fx/fx) mice were also bred with Col1a1-Cre transgenic mice to produce osteoblast-specific Smad1 cKO mice. Postnatal bone formation was assessed by micro-computed tomography (μCT) and histological analyses in 2-month-old mice. Mineralized bone nodule formation assay, 5-bromo-2'-deoxy-uridine (BrdU) labeling and gene expression analysis were performed.
Results: Mice with chondrocyte- and osteoblast-specific deletion of the Smad1 gene are viable and fertile. Calvarial bone development was delayed in chondrocyte-specific Smad1 cKO mice. In osteoblast-specific Smad1 cKO mice, BMP signaling was partially inhibited and mice developed an osteopenic phenotype. Osteoblast proliferation and differentiation were impaired in osteoblast-specific Smad1 cKO mice.
Conclusions: Smad1 plays an essential role in bone development and postnatal bone formation.
Copyright © 2011 Osteoarthritis Research Society International. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures





Similar articles
-
SMAD1/5 signaling in osteoclasts regulates bone formation via coupling factors.PLoS One. 2018 Sep 6;13(9):e0203404. doi: 10.1371/journal.pone.0203404. eCollection 2018. PLoS One. 2018. PMID: 30188920 Free PMC article.
-
Runx1 is a central regulator of osteogenesis for bone homeostasis by orchestrating BMP and WNT signaling pathways.PLoS Genet. 2021 Jan 21;17(1):e1009233. doi: 10.1371/journal.pgen.1009233. eCollection 2021 Jan. PLoS Genet. 2021. PMID: 33476325 Free PMC article.
-
Conditional Alpl Ablation Phenocopies Dental Defects of Hypophosphatasia.J Dent Res. 2017 Jan;96(1):81-91. doi: 10.1177/0022034516663633. Epub 2016 Oct 1. J Dent Res. 2017. PMID: 27582029 Free PMC article.
-
Mechanisms involved in bone resorption regulated by vitamin D.J Steroid Biochem Mol Biol. 2018 Mar;177:70-76. doi: 10.1016/j.jsbmb.2017.11.005. Epub 2017 Nov 14. J Steroid Biochem Mol Biol. 2018. PMID: 29146302 Review.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
Cited by
-
MMP13 is a critical target gene during the progression of osteoarthritis.Arthritis Res Ther. 2013 Jan 8;15(1):R5. doi: 10.1186/ar4133. Arthritis Res Ther. 2013. PMID: 23298463 Free PMC article.
-
Correlation of high urinary Smad1 level with glomerular hyperfiltration in type 2 diabetes mellitus.Endocrine. 2013 Apr;43(2):346-50. doi: 10.1007/s12020-012-9741-9. Epub 2012 Jul 14. Endocrine. 2013. PMID: 22798249
-
miR‑203‑3p participates in the suppression of diabetes‑associated osteogenesis in the jaw bone through targeting Smad1.Int J Mol Med. 2018 Mar;41(3):1595-1607. doi: 10.3892/ijmm.2018.3373. Epub 2018 Jan 9. Int J Mol Med. 2018. PMID: 29328402 Free PMC article.
-
Reduced APPL1 impairs osteogenic differentiation of mesenchymal stem cells by facilitating MGP expression to disrupt the BMP2 pathway in osteoporosis.J Biol Chem. 2023 Jun;299(6):104823. doi: 10.1016/j.jbc.2023.104823. Epub 2023 May 13. J Biol Chem. 2023. PMID: 37187293 Free PMC article.
-
Specification of BMP Signaling.Cells. 2019 Dec 5;8(12):1579. doi: 10.3390/cells8121579. Cells. 2019. PMID: 31817503 Free PMC article. Review.
References
-
- Shum L, Coleman CM, Hatakeyama Y, Rocky TS. Morphogenesis and dysmorphogenesis of the appendicular skeleton. Birth Defects Res C Embryo Today. 2003;69:102–22. - PubMed
-
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–54. - PubMed
-
- Zelzer E, Olsen BR. The genetic basis for skeletal diseases. Nature. 2003;423:343–8. - PubMed
-
- Yang X, Karsenty G. Transcription factors in bone: developmental and pathological aspects. Trends Mol Med. 2002;8:340–5. - PubMed
-
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

