The double-length tyrosyl-tRNA synthetase from the eukaryote Leishmania major forms an intrinsically asymmetric pseudo-dimer
- PMID: 21420975
- PMCID: PMC3095712
- DOI: 10.1016/j.jmb.2011.03.026
The double-length tyrosyl-tRNA synthetase from the eukaryote Leishmania major forms an intrinsically asymmetric pseudo-dimer
Abstract
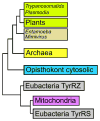
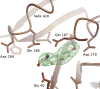
The single tyrosyl-tRNA synthetase (TyrRS) gene in trypanosomatid genomes codes for a protein that is twice the length of TyrRS from virtually all other organisms. Each half of the double-length TyrRS contains a catalytic domain and an anticodon-binding domain; however, the two halves retain only 17% sequence identity to each other. The structural and functional consequences of this duplication and divergence are unclear. TyrRS normally forms a homodimer in which the active site of one monomer pairs with the anticodon-binding domain from the other. However, crystal structures of Leishmania major TyrRS show that, instead, the two halves of a single molecule form a pseudo-dimer resembling the canonical TyrRS dimer. Curiously, the C-terminal copy of the catalytic domain has lost the catalytically important HIGH and KMSKS motifs characteristic of class I aminoacyl-tRNA synthetases. Thus, the pseudo-dimer contains only one functional active site (contributed by the N-terminal half) and only one functional anticodon recognition site (contributed by the C-terminal half). Despite biochemical evidence for negative cooperativity between the two active sites of the usual TyrRS homodimer, previous structures have captured a crystallographically-imposed symmetric state. As the L. major TyrRS pseudo-dimer is inherently asymmetric, conformational variations observed near the active site may be relevant to understanding how the state of a single active site is communicated across the dimer interface. Furthermore, substantial differences between trypanosomal TyrRS and human homologs are promising for the design of inhibitors that selectively target the parasite enzyme.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

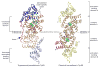

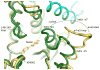





Similar articles
-
Crystal structure of a human aminoacyl-tRNA synthetase cytokine.Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15369-74. doi: 10.1073/pnas.242611799. Epub 2002 Nov 11. Proc Natl Acad Sci U S A. 2002. PMID: 12427973 Free PMC article.
-
Structural snapshots of the KMSKS loop rearrangement for amino acid activation by bacterial tyrosyl-tRNA synthetase.J Mol Biol. 2005 Feb 11;346(1):105-17. doi: 10.1016/j.jmb.2004.11.034. Epub 2004 Dec 15. J Mol Biol. 2005. PMID: 15663931
-
Tryptophanyl-tRNA synthetase crystal structure reveals an unexpected homology to tyrosyl-tRNA synthetase.Structure. 1995 Jan 15;3(1):17-31. doi: 10.1016/s0969-2126(01)00132-0. Structure. 1995. PMID: 7743129
-
Discrimination between transfer-RNAs by tyrosyl-tRNA synthetase.Biochimie. 1993;75(12):1099-108. doi: 10.1016/0300-9084(93)90009-h. Biochimie. 1993. PMID: 8199245 Review.
-
Non-canonical functions of human cytoplasmic tyrosyl-, tryptophanyl- and other aminoacyl-tRNA synthetases.Enzymes. 2020;48:207-242. doi: 10.1016/bs.enz.2020.04.001. Epub 2020 Jun 12. Enzymes. 2020. PMID: 33837705 Review.
Cited by
-
Bioinformatic Analysis Reveals Archaeal tRNATyr and tRNATrp Identities in Bacteria.Life (Basel). 2017 Feb 21;7(1):8. doi: 10.3390/life7010008. Life (Basel). 2017. PMID: 28230768 Free PMC article.
-
Aminoacyl-tRNA synthetases as drug targets in eukaryotic parasites.Int J Parasitol Drugs Drug Resist. 2013 Nov 11;4(1):1-13. doi: 10.1016/j.ijpddr.2013.10.001. eCollection 2014 Apr. Int J Parasitol Drugs Drug Resist. 2013. PMID: 24596663 Free PMC article. Review.
-
New molecular insights into the tyrosyl-tRNA synthase inhibitors: CoMFA, CoMSIA analyses and molecular docking studies.Sci Rep. 2017 Sep 14;7(1):11525. doi: 10.1038/s41598-017-10618-1. Sci Rep. 2017. PMID: 28912450 Free PMC article.
-
Comparison of histidine recognition in human and trypanosomatid histidyl-tRNA synthetases.Biochimie. 2014 Nov;106:111-20. doi: 10.1016/j.biochi.2014.08.005. Epub 2014 Aug 20. Biochimie. 2014. PMID: 25151410 Free PMC article.
-
Aminoacyl tRNA Synthetases: Implications of Structural Biology in Drug Development against Trypanosomatid Parasites.ACS Omega. 2023 Apr 10;8(17):14884-14899. doi: 10.1021/acsomega.3c00826. eCollection 2023 May 2. ACS Omega. 2023. PMID: 37151504 Free PMC article. Review.
References
-
- Eriani G, Delarue M, Poch O, Gangloff J, Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990;347:203–6. - PubMed
-
- Cusack S, Berthet-Colominas C, Hartlein M, Nassar N, Leberman R. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 Å. Nature. 1990;347:249–255. - PubMed
-
- Wolf YI, Aravind L, Grishin NV, Koonin EV. Evolution of aminoacyl-tRNA synthetases–analysis of unique domain architectures and phylogenetic trees reveals a complex history of horizontal gene transfer events. Genome Res. 1999;9:689–710. - PubMed
-
- Brindefalk B, Viklund J, Larsson D, Thollesson M, Andersson SGE. Origin and evolution of the mitochondrial aminoacyl-tRNA synthetases. Mol Biol Evol. 2007;24:743–56. - PubMed
-
- Brown J, Robb F, Weiss R, Doolittle W. Evidence for the early divergence of tryptophanyl- and tyrosyl-tRNA synthetases. J Molec Evol. 1997;45:9–16. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

