WAXS studies of the structural diversity of hemoglobin in solution
- PMID: 21420976
- PMCID: PMC3081904
- DOI: 10.1016/j.jmb.2011.02.062
WAXS studies of the structural diversity of hemoglobin in solution
Abstract
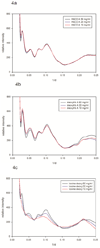
Specific ligation states of hemoglobin are, when crystallized, capable of taking on multiple quaternary structures. The relationship between these structures, captured in crystal lattices, and hemoglobin structure in solution remains uncertain. Wide-angle X-ray solution scattering (WAXS) is a sensitive probe of protein structure in solution that can distinguish among similar structures and has the potential to contribute to these issues. We used WAXS to assess the relationships among the structures of human and bovine hemoglobins in different liganded forms in solution. WAXS data readily distinguished among the various forms of hemoglobins. WAXS patterns confirm some of the relationships among hemoglobin structures that have been defined through crystallography and NMR and extend others. For instance, methemoglobin A in solution is, as expected, nearly indistinguishable from HbCO A. Interestingly, for bovine hemoglobin, the differences between deoxy-Hb, methemoglobin and HbCO are smaller than the corresponding differences in human hemoglobin. WAXS data were also used to assess the spatial extent of structural fluctuations of various hemoglobins in solution. Dynamics has been implicated in allosteric control of hemoglobin, and increased dynamics has been associated with lowered oxygen affinity. Consistent with that notion, WAXS patterns indicate that deoxy-Hb A exhibits substantially larger structural fluctuations than HbCO A. Comparisons between the observed WAXS patterns and those predicted on the basis of atomic coordinate sets suggest that the structures of Hb in different liganded forms exhibit clear differences from known crystal structures.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
The enigma of the liganded hemoglobin end state: a novel quaternary structure of human carbonmonoxy hemoglobin.Biochemistry. 2005 Jun 14;44(23):8347-59. doi: 10.1021/bi050412q. Biochemistry. 2005. PMID: 15938624
-
The X-ray structure determination of bovine carbonmonoxy hemoglobin at 2.1 A resoultion and its relationship to the quaternary structures of other hemoglobin crystal froms.Protein Sci. 2001 Jun;10(6):1091-9. doi: 10.1110/ps.48301. Protein Sci. 2001. PMID: 11369847 Free PMC article.
-
Modulation of hemoglobin dynamics by an allosteric effector.Protein Sci. 2017 Mar;26(3):505-514. doi: 10.1002/pro.3099. Epub 2017 Feb 14. Protein Sci. 2017. PMID: 27977887 Free PMC article.
-
Protein dynamics explain the allosteric behaviors of hemoglobin.Biochim Biophys Acta. 2008 Sep;1784(9):1146-58. doi: 10.1016/j.bbapap.2008.04.025. Epub 2008 May 8. Biochim Biophys Acta. 2008. PMID: 18519045 Free PMC article. Review.
-
The structure--function relationship of hemoglobin in solution at atomic resolution.Chem Rev. 2004 Mar;104(3):1219-30. doi: 10.1021/cr940325w. Chem Rev. 2004. PMID: 15008621 Review. No abstract available.
Cited by
-
Comparative analysis of oxy-hemoglobin and aquomet-hemoglobin by hydrogen/deuterium exchange mass spectrometry.J Am Soc Mass Spectrom. 2013 Jul;24(7):997-1005. doi: 10.1007/s13361-013-0647-4. Epub 2013 May 11. J Am Soc Mass Spectrom. 2013. PMID: 23666601
-
New look at hemoglobin allostery.Chem Rev. 2015 Feb 25;115(4):1702-24. doi: 10.1021/cr500495x. Epub 2015 Jan 21. Chem Rev. 2015. PMID: 25607981 Free PMC article. Review. No abstract available.
-
Wide-angle X-ray solution scattering for protein-ligand binding: multivariate curve resolution with Bayesian confidence intervals.Biophys J. 2013 Feb 19;104(4):873-83. doi: 10.1016/j.bpj.2012.12.019. Biophys J. 2013. PMID: 23442966 Free PMC article.
-
NMR experiments redefine the hemoglobin binding properties of bacterial NEAr-iron Transporter domains.Protein Sci. 2019 Aug;28(8):1513-1523. doi: 10.1002/pro.3662. Epub 2019 Jul 3. Protein Sci. 2019. PMID: 31120610 Free PMC article.
-
Sulfmyoglobin Conformational Change: A Role in the Decrease of Oxy-Myoglobin Functionality.Biochem Biophys Rep. 2016 Sep;7:386-393. doi: 10.1016/j.bbrep.2016.07.002. Epub 2016 Jul 7. Biochem Biophys Rep. 2016. PMID: 28138567 Free PMC article.
References
-
- Perutz MF. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970;228:726–739. - PubMed
-
- Park S-Y, Yokoyama T, Shibayama N, Shiro Y, Tame JRH. 1.25 Å Resolution Crystal Structures of Human Haemoglobin in the Oxy, Deoxy and Carbonmonoxy Forms. J. Mol. Biol. 2006;360:690–701. - PubMed
-
- Smith FR, Lattman EE, Carter CW., Jr The mutation α99 Asp-Tyr stabilizes Y: A new, composite quaternary state of human hemoglobin. Proteins. 1991;10:81–91. - PubMed
-
- Silva MM, Rogers PH, Arnone A. A third quaternary structure of human hemoglobin A at 1.7-A resolution. J Biol Chem. 1992;267:17248–17256. - PubMed
-
- Tame J. What is the true structure of liganded hemoglobin. Trends Biochem. 1999;24:372–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

