Electrophysiologic basis for the antiarrhythmic actions of ranolazine
- PMID: 21421082
- PMCID: PMC3131428
- DOI: 10.1016/j.hrthm.2011.03.045
Electrophysiologic basis for the antiarrhythmic actions of ranolazine
Abstract
Ranolazine is a Food and Drug Administration-approved antianginal agent. Experimental and clinical studies have shown that ranolazine has antiarrhythmic effects in both ventricles and atria. In the ventricles, ranolazine can suppress arrhythmias associated with acute coronary syndrome, long QT syndrome, heart failure, ischemia, and reperfusion. In atria, ranolazine effectively suppresses atrial tachyarrhythmias and atrial fibrillation (AF). Recent studies have shown that the drug may be effective and safe in suppressing AF when used as a pill-in-the pocket approach, even in patients with structurally compromised hearts, warranting further study. The principal mechanism underlying ranolazine's antiarrhythmic actions is thought to be primarily via inhibition of late I(Na) in the ventricles and via use-dependent inhibition of peak I(Na) and I(Kr) in the atria. Short- and long-term safety of ranolazine has been demonstrated in the clinic, even in patients with structural heart disease. This review summarizes the available data regarding the electrophysiologic actions and antiarrhythmic properties of ranolazine in preclinical and clinical studies.
Copyright © 2011 Heart Rhythm Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest: Dr. Antzelevitch received a research grant and serves as a consultant to Gilead Sciences and Dr. Belardinelli is employed by Gilead Sciences.
Figures
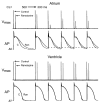



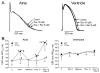
Comment in
-
Ranolazine: Déjà vu of the amiodarone story.Heart Rhythm. 2011 Aug;8(8):1291-2. doi: 10.1016/j.hrthm.2011.06.007. Epub 2011 Jun 7. Heart Rhythm. 2011. PMID: 21699867 No abstract available.
References
-
- Song Y, Shryock JC, Wu L, et al. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2004;44:192–9. - PubMed
-
- Sossalla S, Kallmeyer B, Wagner S, et al. Altered Na+ currents in atrial fibrillation: effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol. 2010;55:2330–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

